#glutamate
“It was like red-hot pokers needling one side of my face,” says Catherine, recalling the cluster headaches she experienced for six years. “I just wanted it to stop.” But it wouldn’t – none of the drugs she tried had any effect.

Thinking she had nothing to lose, last year she enrolled in a pilot study to test a handheld device that applies a bolt of electricity to the neck, stimulating the vagus nerve – the superhighway that connects the brain to many of the body’s organs, including the heart.
The results of the trial were presented last month at the International Headache Congress in Boston, and while the trial is small, the findings are positive. Of the 21 volunteers, 18 reported a reduction in the severity and frequency of their headaches, rating them, on average, 50 per cent less painful after using the device daily and whenever they felt a headache coming on.
This isn’t the first time vagal nerve stimulation has been used as a treatment – but it is one of the first that hasn’t required surgery. Some people with epilepsy have had a small generator that sends regular electrical signals to the vagus nerve implanted into their chest. Implanted devices have also been approved to treat depression. What’s more, there is increasing evidence that such stimulation could treat many more disorders from headaches to stroke and possibly Alzheimer’s disease.
The latest study suggests it is possible to stimulate the nerve through the skin, rather than resorting to surgery. “What we’ve done is figured out a way to stimulate the vagus nerve with a very similar signal, but non-invasively through the neck,” says Bruce Simon, vice-president of research at New Jersey-based ElectroCore, makers of the handheld device. “It’s a simpler, less invasive way to stimulate the nerve.”
Cluster headaches are thought to be triggered by the overactivation of brain cells involved in pain processing. The neurotransmitter glutamate, which excites brain cells, is a prime suspect. ElectroCore turned to the vagus nerve as previous studies had shown that stimulating it in people with epilepsy releases neurotransmitters that dampen brain activity.
When the firm used a smaller version of ElectroCore’s device on rats, it found it reduced glutamate levels and excitability in these pain centres. Other studies have shown that vagus nerve stimulation causes the release of inhibitory neurotransmitters which counter the effects of glutamate.
The big question is whether a non-implantable device can really trigger changes in brain chemistry in humans, or whether people are simply experiencing a placebo effect. “The vagus nerve is buried deep in the neck, and something that’s delivering currents through the skin can only go so deep,” says Mike Kilgard of the University of Texas at Dallas. As you turn up the voltage, there’s a risk of it activating muscle fibres that trigger painful cramps, he adds.
Simon says that volunteers using the device haven’t reported any serious side effects. He adds that ElectroCore will soon publish data showing changes in brain activity in humans after using the device. Placebo-controlled trials are also about to start.
Catherine has been using it for a year without ill effect. “I can now function properly as a human being again,” she says.
The many uses of the wonder nerve
Coma, irritable bowel syndrome, asthma and obesity are just some of the disparate conditions that vagus nerve stimulation may benefit and for which human trials are under way.
It might also help people with tinnitus. Although people with tinnitus complain of ringing in their ears, the problem actually arises because too many neurons fire in the auditory part of the brain when certain frequencies are heard.
Mike Kilgard of the University of Texas at Dallas reasoned that if people were played tones that didn’t trigger tinnitus while the vagus nerve was stimulated, this might coax the rogue neurons into firing in response to these frequencies instead. “By activating this nerve we can enhance the brain’s ability to rewire itself,” he says.
He has so far tested the method in rats and in 10 people with tinnitus, using an implanted device to stimulate the nerve. Not everyone noticed an improvement, but even so Kilgard is planning a larger trial. The work was presented at a meeting of the International Union of Physiological Sciences in Birmingham, UK, last month. The technique is also being tested in people who have had a stroke.
“If these studies stand up it could be worth changing the name of the vagus nerve to the wonder nerve,” says Sunny Ogbonnaya at Cork University Hospital in Ireland.
New findings on how ketamine acts against depression
The discovery that the anaesthetic ketamine can help people with severe depression has raised hopes of finding new treatment options for the disease. Researchers at Karolinska Institutet have now identified novel mechanistic insights of how the drug exerts its antidepressant effect. The findings have been published in the journal Molecular Psychiatry.
According to the World Health Organization, depression is a leading cause of disability worldwide and the disease affects more than 360 million people every year.
The risk of suffering is affected by both genetics and environmental factors. The most commonly prescribed antidepressants, such as SSRIs, affect nerve signalling via monoamines in the brain.
However, it can take a long time for these drugs to help, and over 30 percent of sufferers experience no relief at all.
The need for new types of antidepressants with faster action and wider effect is therefore considerable.
An important breakthrough is the anaesthetic ketamine, which has been registered for some years in the form of a nasal spray for the treatment of intractable depression.
Relieves depressive symptoms quickly
Unlike classic antidepressants, ketamine affects the nerve signalling that occurs via the glutamate system, but it is unclear exactly how the antidepressant effect is mediated. When the medicine has an effect, it relieves depressive symptoms and suicidal thoughts very quickly.
However, ketamine can cause unwanted side effects such as hallucinations and delusions and there may be a risk of abuse so alternative medicines are needed.
The researchers want to better understand how ketamine works in order to find substances that can have the same rapid effect but without the side effects.
Explains ketamine’s effects
In a new study, researchers at Karolinska Institutet have further investigated the molecular mechanisms underlying ketamine’s antidepressant effects. Using experiments on both cells and mice, the researchers were able to show that ketamine reduced so-called presynaptic activity and the persistent release of the neurotransmitter glutamate.
“Elevated glutamate release has been linked to stress, depression and other mood disorders, so lowered glutamate levels may explain some of the effects of ketamine,” says Per Svenningsson, professor at the Department of Clinical Neuroscience, Karolinska Institutet, and the study’s last author.
When nerve signals are transmitted, the transmission from one neuron to the next occurs via synapses, a small gap where the two neurons meet.
The researchers were able to see that ketamine directly stimulated AMPA receptors, which sit postsynaptically, that is, the part of the nerve cell that receives signals and this leads to the increased release of the neurotransmitter adenosine which inhibits presynaptic glutamate release.
The effects of ketamine could be counteracted by the researchers inhibiting presynaptic adenosine A1 receptors.
“This suggests that the antidepressant action of ketamine can be regulated by a feedback mechanism. It is new knowledge that can explain some of the rapid effects of ketamine,” says Per Svenningsson.
In collaboration with Rockefeller University, the same research group has also recently reported on the disease mechanism in depression.
The findings, also published in the journal Molecular Psychiatry, show how the molecule p11 plays an important role in the onset of depression by affecting cells sitting on the surface of the brain cavity, ependymal cells, and the flow of cerebrospinal fluid.
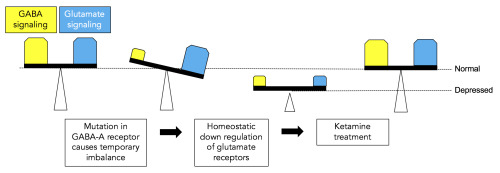
(Image caption: Mutations in the GABA-A receptor cause a temporary imbalance between GABA signaling (yellow) and glutamate signaling (blue) in the brain. Homeostatic down regulation of glutamate signaling rebalances the system at a lower level and can lead to depression. Treatment with ketamine restores both GABA and glutamate levels to normal. Credit: Penn State University)
New research demonstrates the effectiveness of ketamine to treat depression in a mouse model of the disease and brings together two hypotheses for the cause of depression. The research, led by Bernhard Lüscher, professor of biology and of biochemistry and molecular biology at Penn State University, is in press and was published in the September 15, 2016 print edition of the journal Biological Psychiatry.
“Depression is the second most expensive health problem that we face worldwide, but this fact is not very well known because there is a stigma attached to depression and people don’t like to talk about it,” said Lüscher. “About 17 percent of Americans will be treated for depression at some point in their lives, but there are limited treatment options and about one-third of patients do not respond to these treatments.”
Lüscher and his colleagues generated a mouse model for depression by introducing a mutation into a gene that codes for one of the subunits of a receptor for GABA – the second most abundant chemical used by nerve cells in the brain to communicate. GABA functions mainly to reduce the activity of nerve cells. The receptor mutation results in a reduction in GABA signaling of about 15 to 20 percent and mimics reductions in GABA signaling seen in patients with depression. The mice that have the mutation exhibit traits associated with depression, such as reduced pleasure seeking, and they become normal again following treatment with antidepressant medications.
“You can think of GABA as acting like the brakes of a car – its function is to slow activity in nerve cells,” said Lüscher. “Its counterpart is glutamate, another signaling chemical in the brain that acts as the accelerator of nerve-cell activity. When we reduced the function of GABA in our mice, we were surprised to see that the level of glutamate was also reduced. This result suggests that the brain has mechanisms that maintain a balance between the brakes and the accelerator to prevent brain activity from going out of control, a state we refer to as homeostasis.”
The researchers treated the mice with low doses of ketamine, an experimental antidepressant drug known to act by transiently blocking a major class of glutamate receptors in nerve cells. “Treatment with ketamine not only normalized the behavior and brought glutamate receptor levels back up to normal in our mice, but GABA function also was restored,” said Lüscher. Importantly, the effects of ketamine were only observed in mice with the receptor mutation where nerve signaling was defective, and not in normal mice. “Our results bring together the hypothesis that depression results from deficits in GABA signaling and the hypothesis that depression results from deficits in glutamate signaling. We showed that the depression-like behavior in our mice results from the reduction of both GABA and glutamate, and importantly, that both can be restored with a single dose of ketamine.”
The researchers plan to use their mouse model to better understand how ketamine functions to develop safer alternatives. “Ketamine has many advantages over currently used antidepressant medications,” said Lüscher. “It acts quickly and has long lasting effects, but it is addictive and can induce psychosis, so we hope to use our model to better understand how ketamine works biochemically. We can then begin to develop ketamine-like drugs without the unwanted side effects.”
Post link
In a new study published in Applied Physiology, Nutrition, and Metabolism, scientists from the University of Guelph have found that exercise has the potential to decrease toxic build-up in the brain, reducing the severity of brain disorders such as Huntington’s disease.
Glutamate, an amino acid that is one of the twenty amino acids used to construct proteins, is used by the brain to transmit signals, but too much glutamate blocks future signals and can lead to toxicity in the brain. Since the majority of the brain relies on glutamate as the main neurotransmitter for communication between neural cells, it is essential that glutamate is reabsorbed and disposed of to prevent blockage. While glutamate reuptake is a normal process for healthy brains, several diseases such as Huntington’s disease, ALS, and epilepsy result in either failed reuptake of glutamate or high levels of glutamate in the brain. This can lead to unwanted and in some cases excessive stimulation of neighbouring cells which can worsen the disease.
The findings of this study show that exercise has the potential to increase the use of glutamate in the brain and may help reduce the toxicity caused by glutamate build-up in these diseases. “As we all know, exercise is healthy for the rest of the body and our study suggests that exercise may present an excellent option for reducing the severity of brain disorders” says Dr. Eric Herbst, lead author of the study. “Taking into account that there are no cures for neurodegenerative diseases where glutamate is implicated, this study offers another example of the benefits of exercise for our brains” continued Dr. Herbst. “In short, these findings offer another reason to exercise with the aim of either preventing or slowing the neurodegeneration caused by these disorders”.
The findings of this study are of particular importance to other researchers exploring different approaches to treating brain disorders. The main approaches to treating neurodegenerative diseases are hindered by the need to produce drugs that both have the intended effect for treating the disease and are also able to pass the blood brain barrier. Through the use of exercise, the brain can direct glutamate to be used as an energy source to dispose of excess amounts of the neurotransmitter, without relying on the difficult development of pharmaceuticals. Identifying and targeting the mechanisms that increase glutamate metabolism in the brain may also provide the medical field with additional ways of treating problems within the brain. How the findings of this study translates to people affected by neurodegenerative diseases still needs exploring and is an important next step.