#synapses
IST Austria Professor Peter Jonas and team identify a new learning rule.
“Fire together, wire together” is the famous abridged version of the Hebbian rule. It states that neurons in the brain adapt during the learning process, a mechanism which is called neuronal plasticity. Hebb’s theory dates back to the 1940s and subsequent research in neuroscience has further corroborated it. Today, we also know that different factors play a critical role, such as timing of firing, order of activity, and functional connectivity, as cutting-edge technologies allow examining subcellular processes with extraordinary precision.
Recently, scientists at the Institute of Science and Technology Austria (IST Austria) discovered a new learning rule for a specific type of excitatory synaptic connection in the hippocampus. Their study was now published in the renowned journal Nature Communications on May 13. These synapses are located in the so-called CA3 region of the hippocampus, which plays a critical role for storage and recall of spatial information in the brain. One of its hallmark properties is that memory recall can even be triggered by incomplete cues. This enables the network to complete neuronal activity patterns, a phenomenon termed pattern completion.
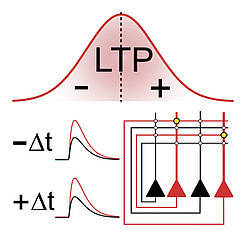
Professor Peter Jonas and his team, including postdoc José Guzmán and PhD student Rajiv Mishra, investigated how the strength of connections between neurons is adjusted, taking into account the relative timing of firing neurons. In neuroscience, this is known as spike-timing-dependent plasticity or STDP. According to the STDP rule, neuron A has to fire just before neuron B so that the synaptic connection becomes stronger with time. In the case of a reverse order—neuron B fires before neuron A—the connection between the neurons may become weaker.
Yet in apparent contrast to this rule, the team of Professor Jonas discovered in their experiments that a reverse order also leads to stronger connections between the investigated synapses (CA3–CA3 recurrent excitatory synapses). Surprisingly, a potentiation takes place independent of the order of firing. So if the sequence is not important at these particular synapses, why is this the case?
To address this question, the authors performed various cutting-edge measurements with extremely high precision. These included patch-clamp recordings to control which neurons fire at what time, imaging of calcium molecules, which play a critical role in synaptic plasticity, and subcellular recordings of electrical signals in dendrites. All data resulted in the same symmetric summation curves. Thus, the unusual induction curve of potentiation is generated by the properties of calcium signaling, which is in turn explained by the characteristics of electrical signaling in dendrites.
The scientists subsequently investigated what happens if a huge number of neurons is being connected via excitatory synapses in a network model. To this end, they ran computer simulations after incorporating different plasticity induction rules. They compared the results of simulations with the new symmetric plasticity induction rule with those of a conventional rule. The outcome clearly demonstrated that patterns could be better restored from partial cues when the new symmetric rule was applied. Professor Jonas: “The new plasticity induction rule may explain why learning in vivo occurs robustly under a variety of behavioral conditions. For example, it may explain storage and recall of cell assembly patterns of freely moving animals in open fields, as previously found by the systems neuroscience groups of IST Austria (O’Neill et al., 2008)”.
The new data seem to be in contrast to classical STDP induction rules at other glutamatergic synapses. Do they violate the Hebb rule? Professor Jonas: “If you read the classical Hebb text carefully, it states: ‘If the axon of a cell A is near enough to excite cell B […], A’s efficiency, as one of the cells firing B, is increased’. However, there is no mentioning of depression. So the new data do not violate Hebb’s postulate, but may confirm it in the literal sense”.

Call-and-response circuit tells neurons when to grow synapses
Brain cells called astrocytes play a key role in helping neurons develop and function properly, but there’s still a lot scientists don’t understand about how astrocytes perform these important jobs. Now, a team of scientists led by Associate Professor Nicola Allen has found one way that neurons and astrocytes work together to form healthy connections called synapses. This insight into normal astrocyte function could help scientists better understand disorders linked to problems with neuronal development, including autism spectrum disorders. The study was published in the journal eLife.
“We know that astrocytes could play a role in neurodevelopmental disorders, so we wanted to ask: How are they playing a role in typical development?” says Allen, a member of the Molecular Neurobiology Laboratory. “In order to better understand the disorders, we first have to understand what happens normally.”
Synapses form critical connections between neurons, allowing neurons to send signals and information throughout the body. Astrocyte cells play a role in synapse development by giving neurons directions, such as telling them when to start growing a synapse, when to stop, when to prune it back, and when to stabilize the connection.
Allen and her team took a closer look at how this process plays out in the visual cortex of the mouse brain. They sequenced the RNA of astrocytes at different stages of brain development to assess gene activity and compared it with neuronal synapse development. They found that astrocyte signaling was directly related to each stage of neuronal development. The researchers then wanted to know how the astrocytes knew to make these signals at the right time.
First, the researchers looked at what happened to the astrocytes when they changed the neurons’ activity. To do this, they stopped neurons from releasing a neurotransmitter called glutamate that can signal to astrocytes, and this stopped the astrocytes from showing the typical developmental changes. Next, the scientists stopped the astrocytes from responding to neurotransmitters, and found this stopped the astrocytes from expressing the right signals. With both these manipulations, the development of synapses was also disrupted, in line with the changes observed in the astrocytes.
Collectively, the findings suggest that astrocytes are responding to neurotransmitters produced by neurons to control the timing of when astrocytes produce signals to instruct neuronal development, according to Allen.
“It makes sense that you have this constant feedback going on between the neuron and the astrocyte,” says Allen. “They are sending signals to each other: ‘Am I in the right place?’ ‘Yes, you are.’ ‘I’ve made a connection now—do I keep it?’ ‘Yes, you do.’ And they keep going back and forth.”
Next, Allen and her team are studying whether these signals can be manipulated—for example, to stimulate neurons to repair synapses or form new ones in disorders of aging, such as Alzheimer’s disease.
(Image caption: Astrocytes (green) and neurons (magenta) closely interact in the developing cortex and signal to each other to ensure correct development. Credit: Salk Institute)

Researchers at the University of Bonn have shed light on the function of the enzyme SLK for the development of nerve cells in the brain. If it is missing, the neurons’ branches are less abundant. In addition, it is then more difficult to inhibit the activity of the cells. This is consistent with the fact that there is less SLK in diseased brain tissue from epilepsy patients. Epileptic seizures are characterized by overexcitation of neuron clusters. The findings may help to improve treatment of the disease. The study is published in the prestigious Journal of Neuroscience.
SLK belongs to the large group of kinases. These enzymes are extremely important: They attach phosphate groups (which are small molecular residues with a phosphorus atom in the center) to proteins and thus alter their activity. Kinases are involved in the regulation of almost all life processes in animals.
The kinase SLK was already known to play an important role in embryonic development: One of its effects is on the growth of cells and their migration in the body; these processes are also essential for the maturation of neurons. “We therefore investigated what function SLK performs in nerve cells,” explains Prof. Dr. Albert Becker from the Institute of Neuropathology at the University of Bonn.
The researchers inhibited the production of the SLK protein in neurons of mice. “This changed the appearance of the neurons,” says Anne Quatraccioni, who is doing her doctorate at the Institute of Neuropathology in the research group of Prof. Dr. Susanne Schoch McGovern: “The dendrites, which are the extensions that receive signals from other neurons and conduct them to the cell body, branched less.”
SLK deficiency makes neurons more excitable
The dendrites resemble a kind of tree dotted with tiny contact points, the synapses. This is where extensions of other nerve cells dock and transmit electrical impulses to the tree. The observed “thinning” did not affect the thick main branches, but exclusively the smallest shoots. The synapses on these small branches are called excitatory: Signals received there have an arousing effect. This means that they increase the probability that the neuron will in turn generate an electrical signal, in other words, that it will “fire”.
When there are fewer side branches, the synapses could concentrate in a smaller area and thereby gain influence, making the neuron easier to excite (since the synapses are excitatory). “Surprisingly, however, we did not find an increased density of excitatory synapses,” Quatraccioni points out. “Nevertheless, the affected neurons were more excitable. But there had to be other reasons.”
The cause is not to be found in the delicate twigs, but in the thick main branches. Numerous synapses are also located there, but of a different type: They have an inhibitory effect. Any signal received by these synapses prevents the nerve cell from firing. “The mice initially formed a normal amount of these inhibitory synapses,” Quatraccioni explains. “However, after a few days of life, their density began to decrease. This loss kept progressing.”
SLK therefore appears to be important in maintaining normal levels of inhibitory synapses. Without the kinase, the affected neurons become increasingly difficult to inhibit over time. This fits in with the fact that the researchers were able to detect SLK deficiency in the nerve cells of brain tissue from epilepsy patients. During epileptic seizures, whole areas of the brain are overexcited, meaning that the neurons fire too easily.
Explanation for declining drug efficacy?
The findings could also explain why the effects of the drugs diminish over time in some sufferers. “Perhaps this effect is not due to resistance to the drugs, but to the progressive loss of the inhibitory synapses,” says Prof. Dr. Susanne Schoch McGovern. The findings therefore provide new insights into how the disease develops.
They could also have therapeutic relevance: “We often try to prevent neuronal overexcitation with drugs that stimulate inhibitory synapses,” explains Schoch McGovern. “This might be the wrong strategy in the case of an SLK deficiency: At some point, there are so few inhibitory synapses left that this no longer works. It is probably more promising in these patients to intervene on the excitatory side, that is, to inhibit the excitatory synapses.”
(Image caption: Nerve cells from mice - with normal (top) and reduced SLK expression (bottom). Without SLK, dendrites branch less; moreover, the number of inhibitory synapses (green) decreases. Credit: © Institute of Neuropathology/University of Bonn)
Clashing synapses
.
.
.
#blackandgrey #blackandwhite #bwphotography #abstractart #abstract #trippyartwork #trippy #neurons #synapses #tree #naturephotography #natureatitsbest #collageart #collage #photography #opart #opticalart #monochrome #perfectnature #repetition #repeatingnature
https://www.instagram.com/p/B0bI3W1iqN2/?igshid=1o9nv4fz80ty7
Post link


