#thermal physics

When seeing this picture you may think wow that’s gotta’ be painful! and you’re right! It is indeed painful but it’s bearable. Why?
Well, pressure is defined as the ratio of force to the area over which that force is distributed.
P = F/A
One can also say that pressure is force per unit applied in a direction perpendicular to the surface of an object.
Looking back at the picture, we can see that the area is spread out enough so she can balance. If we pressed one single finger down on one spike, it would hurt much more than when we use our whole hand. Then again, both of those options would be painful and probably not the brightest thing to do!
‘Pressure’ is a key term in Thermal Physics, along with ‘Temperature’ and ‘Heat’. Thermal physics is the combined study of thermodynamics, statistical mechanics and kinetic theory.
We’re pretty much obsessed with Temperature, it’s an important part of our lives. I don’t know about you, but when I wake up to see it’s -10 Celsius outside it certainly makes me grumble. We’re so used to just looking at a thermometer that we might not stop and just wonder, howdoes that actually work?
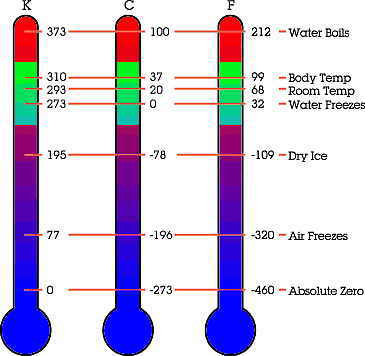
Well, when two objects are in contact with each other, they will eventually both have the same temperature. This can be explained by the zeroth law of thermodynamics/Law of equal temperatures: An object that cannot change it’s own temperature, will eventually reach the same temperature as its surroundings. This is the idea behind our thermometers.
If you’re not familiar with Celsius than you may be familiar with Fahrenheit instead. However, most of us, if not all, are familiar with Kelvin. Absolute zero is defined as a temperature of 0 Kelvin, which is equal to -273.15 Celsius or -459.67 Fahrenheit.
So let’s say you’re more fortunate than I am, with a thermometer that shows a higher temperature. You might say you’re “warm”. In Physics this word is used differently, it’d be more accurate to say: “I’ve got too much internal energy right now!“ In my case it’d be "I really really reaaaallly need more internal energy, help”.
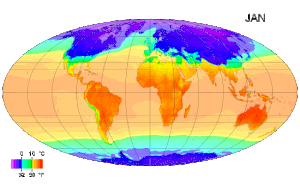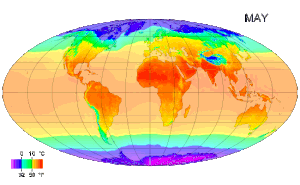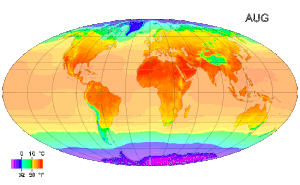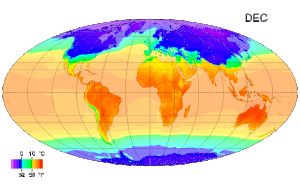
Picture above: A map of global long term monthly average surface air temperature.
What CAN we say about heat, what IS heat?
Heat is defined as energy transferred from one body to another by thermal interactions. Heat has the symbol Q and the SI-unit (for those who don’t know what that is: http://en.wikipedia.org/wiki/International_System_of_Units) J for Joule.
Heat flows from systems of higher temperatures to systems of lower temperatures. When two systems come into thermal contact, they then exchange energy through the microscopic interactions of their particles.
Heat is also necessary when we use the first law of thermodynamics. Which states: The increase in internal energy of a closed system is equal to the difference of the heat supplied to the system and the work done by it:
ΔU = Q - W
Now that we’ve established that heat flows from systems of higher temperature to systems of lower temperatures, can it be reversed? That’s where the Second law of Thermodynamics comes in: Heat cannot spontaneously flow from a colder location to a hotter location.
We also know that energy cannot be created nor can it disappear. So what happens? We have different energy qualities. High energy qualities and low energy qualities. The problem is that a low energy quality cannot turn into a high energy quality, what can happen is that energy is used and transformed and after a while turns into energy with a lower quality. It does not disappear but after a while it pretty much becomes unusable. This is one of the theories of the Universes ‘death’, that in many many maaaany years to come, there will not be any high quality energy left, only unusable energy with a too low quality.

Sources:
http://www.stuffintheair.com/thermometerpictures.html
http://science.nationalgeographic.com/science/enlarge/universe-death.html
http://en.wikipedia.org/wiki/Thermodynamics#Laws_of_thermodynamics
http://en.wikipedia.org/wiki/File:MonthlyMeanT.gif
Physics book: H.Aschehough @ Co. [W. Nygaard] 2007
