#californium
Capable of being produced in milligram amounts, californium-252 can be purchased from the US government, produced in the High Flux Isotope Radiator at the Oak Ridge National Laboratory in Tennessee, for the price of $10 per millionth of a gram.
Periodic Videos on YouTube discusses the element californium and its several applications, including metal detectors.
One of the few transuranic elements with practical applications, certain isotopes of californium are known for their abilities as neutron emitters. As such, californium can be used to help start nuclear reactions or as a source of neutrons when using neutron diffractionorneutron spectroscopy. Lawrencium and element 118, now with the preliminary name oganesson, were both created using atoms of californium-249.
Neutrons produced by isotopes of californium are also used as a treatment of certain cervical and brain cancers where other radiation therapy is ineffective as well as educational applications in various analytical techniques, including detection instruments such as fuel rodscanners.
A highly radioactive and therefore hazardous element, californium has no natural biological role. Given the variety of methods of decay exhibited by californium, the health effects from exposure depend upon the isotope being used.
Californium-252 is a very strong neutron emitter and is extremely harmful, however, neutrons produced from this isotope of californium can be used as a treatment for certain cervical and brain cancers when other radiation therapy is ineffective.
For the most part, this element is most dangerous if taken into the body, where it can disrupt the formation of red blood cells by bioaccumulating in skeletal tissue, but californium-249 and californium-251 emit gamma rays as they decay, allowing them to cause tissue damage externally.
Made in and named after the University of California Radiation Laboratory in Berkeley, California, californium was first synthesized by Stanley G. Thompson, Kenneth Street, Jr., Albert Ghiorso, and Glenn T. Seaborg in early 1950.
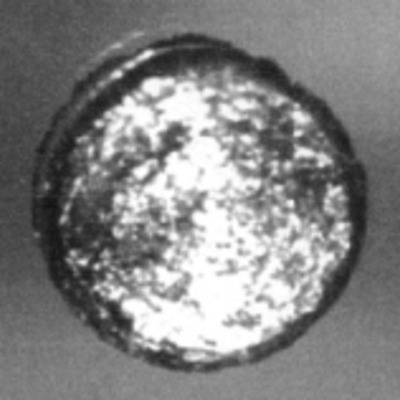
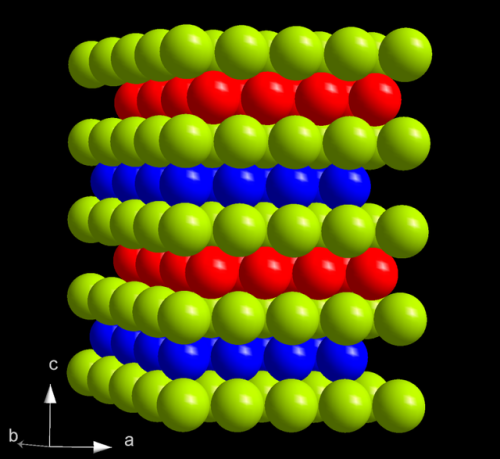
A 1 mm disc of californium next to the crystal structure of its most stable form at ambient conditions, double-hexagonal close packed.
Sources:Californium,Structure
Post link