#viscosity
MIT Engineers Show How Hairy Tongues Help Bats Drink
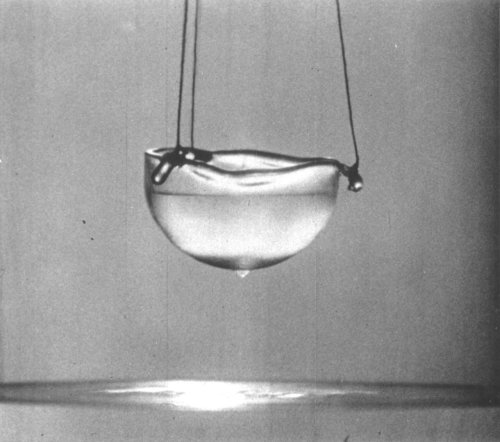
Animals have evolved all manner of adaptations to get the nutrients they need. For nectar-feeding bats, long snouts and tongues let them dip in and out of flowers while hovering in mid-air. To help the cause, their tongues are covered in tiny hairs that serve as miniature spoons to scoop and drag up the tasty sap.
Now engineers at MIT have found that, for bats and other hairy-tongued nectar feeders, the key to drinking efficiently lies in a delicate balance between the spacing of hairs on the tongue, the thickness of the fluid, and the “speed of retraction,” or how fast an animal darts its tongue back to slurp up the nectar. When all three of these parameters are in balance, a good amount of nectar reaches the animal’s mouth instead of dribbling away.
As it happens, the same goes for other hairy-tongued nectar feeders, such as honeybees and honey possums, which the researchers found also exhibit optimal “viscous entrainment,” which refers to the amount of fluid that hairy surfaces can drag up from a bath.
“There are lots of different drinking techniques for animals, and what we think is normal when we drink is really a singular way of drinking,” says Pierre-Thomas Brun, a former instructor in MIT’s Department of Mathematics. “We hope that our theory explains what are the main trending mechanisms of this hairy drinking method, and we believe we have rationalized this peculiar drinking technique.”
Post link
Going superfluid!
A liquid goes superfluid when it suddenly loses all internal friction and gains near infinite thermal conductivity. The combination of zero viscosity but nonzero surface tension allows a superfluid to creep up walls and back down the outside to drip from the bottom of open containers, or to completely cover the inner surface of sealed containers. Lack of viscosity also allows a superfluid to leak through a surface that is porous to any degree, because the molecules can slip through even microscopic holes. Superfluids furthermore exhibit a thermo-mechanical effect where they flow from colder to warmer temperatures, exactly the opposite of heat flow as stated by the laws of thermodynamics! That implies the remarkable property of superfluids of carrying zero entropy. Because of this, a perpetual fountain can be set up by shining light on a superfluid bath just below a vertical open capillary tube, causing the fluid to shoot up through and beyond the tube until its contact with the air causes it to cease being a superfluid and fall back down into the bath, whereby it will cool back into the superfluid state and repeat the process.
So how does superfluidity work, exactly?
Makings of a superfluid
Physicists first got the inkling of something stranger than the norm when, around 1940, they cooled liquid helium (specifically, the 4He isotope) down to 2.17 K and it started exhibiting the above-mentioned properties. Since the chemical makeup of the helium didn’t change (it was still helium), the transformation to a superfluid state is a physical change, a phase transition, just like ice melting into liquid water. Perhaps for cold matter researchers, this transition to a new phase of matter makes up for the fact that helium doesn’t solidify even at 0 K except under large pressure - whereas ALL other substances solidify above 10 K.
 [Phase diagram of 4He,source]
[Phase diagram of 4He,source]
Helium is truly the only substance that never solidifies under its own vapor pressure.
Instead, when the temperature reaches the transition or lambda point, quantum physics takes hold and a fraction of the liquid particles drop into the same ground-energy quantum state. They move in lock-step, behaving identically and never getting in each others’ way. Thus we come to see that superfluidity is a kind of Bose-Einstein condensation, the general phenomenon of a substance’s particles simultaneously occupying the lowest-energy quantum state.
Read more:
“This Month in Physics History: Discovery of Superfluidity, January 1938”.APS News: January 2006
Based on a project by Barbara Bai, Frankie Chan, and Michele Silverstein at Cornell University.
Post link
Glass of Supervicious Fluid
a fine vintage
Take a fucking sip babes
I like how super cruel this fluid is