#buoyancy

Let’s ask a very generic question:
I hand you an object and ask you to predict whether the object would float or sink. How would you go about doing that ?
Well, you can measure the mass of the object and the volume of the object and can derive this quantity called Average Density
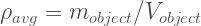
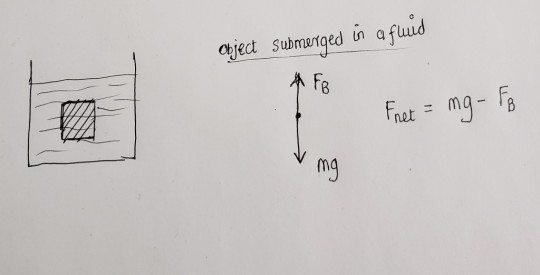
It is the average density of the entire object as a whole. If this object is submerged in a fluid of density , then we can draw the following force diagram:


Fun Experiment:
If you drop some raisins in soda, you will notice that they raise up and fall down like so (Try it out!):
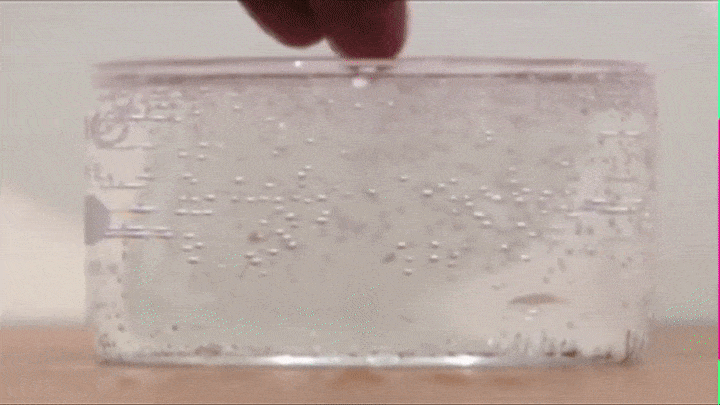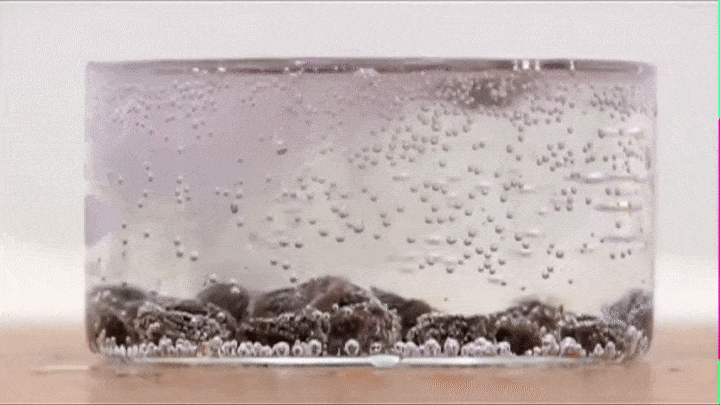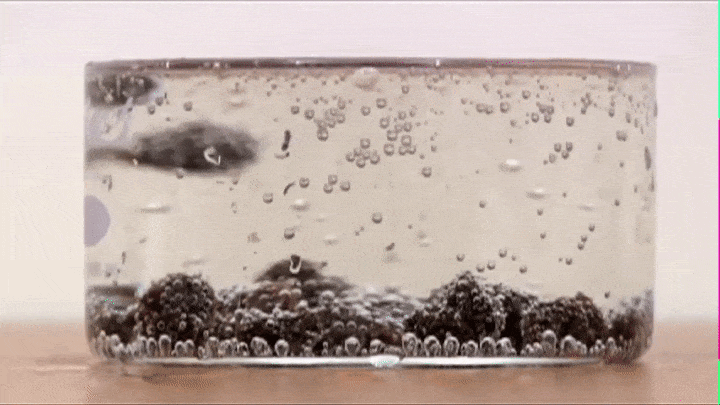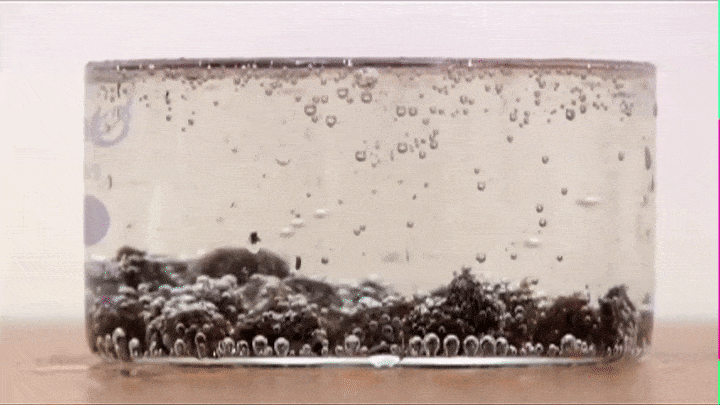
This is because air bubbles that form on the top of the raisin decrease its average density to the point that its able to make the raisin raise all the way from the bottom to the top.
BUT once it reaches the top all the air bubbles escape into the atmosphere (its average density increases) and the raisin now falls down.
Questions to ponder:
- Why do people not sink in the dead sea ?
- How are submarines/divers able to move up and down the ocean ? How would you extend the average density argument in this case.
- Why do air bubbles in soda always want to raise up ?
- If the total load that needs to on a ship is 25 tons. What should be the total volume of the ship in order to remain afloat if the density of sea water is 1029 kg/m3,
** This post is part of the ‘Flow series’ by FYPhysics and EcstasyShots!. Check out the previous posts here.

One of the most surprising things about air that may not be intuitive is that it is a fluid and like any other fluids exerts a pressure on objects.
Standing on earth with layers of air above us, we are constantly being ‘weighed down’ by a pressure of ~1atm at all times.
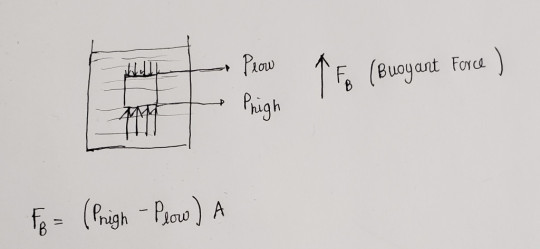
All objects in air are also assisted by a buoyant force that is caused from the pressure difference between the top and bottom surfaces.
Let’s now consider the hot-air balloon in particular. A force diagram is probably the best way to start:

Therefore we see that in order for the hot air balloon to float, we need to have the buoyant force compensate for the net weight from the balloon, the load on it and the air inside it:
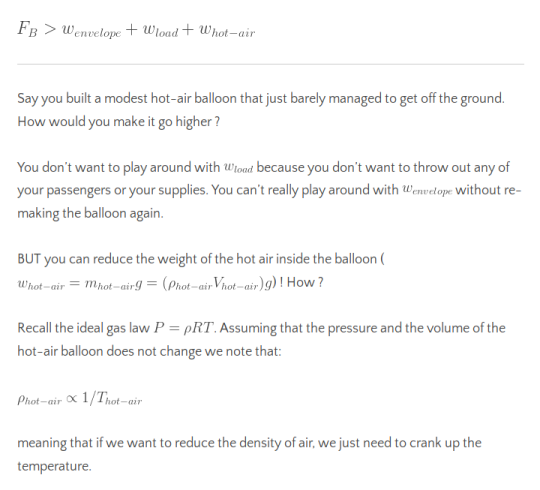

And with a spectacular burner onboard our hot air balloon, we can easily increase the temperature of the air inside the balloon!
That’s pretty much how hot-air balloons work! You increase the temperature of the air inside the balloon to go up, decrease the temperature to go down and skillfully adjust the temperature to hover.
Have a great day!
Questions to ponder:
- When you increase the temperature of air, its density decreases. What do you think happens to all the molecules that were previously inside ? Do they exit the balloon ?
- Different shaped hot-air balloons is a common sight. Do you think that the Buoyant force changes for each shape?
- How do you think Helium balloons work ?
Buoyancy
In the first post of our fluid mechanics series : ‘Flow’ we explore the concept of Buoyancy. Click here to check out the post exploring the physics of Buoyancy.
Have a great day!
* Check out the video description on YouTube on the details of how buoyancy is related to each clip in the video
