#brain development
High blood sugar in young children with type 1 diabetes linked to changes in brain growth
Investigators have found that young children with type 1 diabetes (T1D) have slower brain growth compared to children without diabetes. A new study, published in the December issue of Diabetes, now available ahead of print, suggests that continued exposure to hyperglycemia, or high blood sugars, may be detrimental to the developing brain. The research was supported by the National Institutes of Health (NIH).
“Our results show the potential vulnerability of young developing brains to abnormally elevated glucose levels, even when the diabetes duration has been relatively brief,” said Nelly Mauras, MD, Chief, Division of Endocrinology, Diabetes & Metabolism at Nemours Children’s Clinic in Jacksonville, Fla., and lead author of the study.
Post link
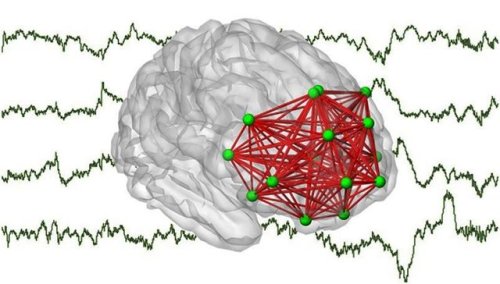
Preterm birth leaves its mark in the functional networks of the brain
Researchers at the University of Helsinki and the Helsinki University Hospital have demonstrated that premature birth has a significant and, at the same time, a very selective effect on the functional networks of a child’s brain. The effects can primarily be seen in the frontal lobe, which is significant for cognitive functions.
Premature birth is globally the most important risk factor for life-time disorders and defects in neurocognitive functions. However, current methods have not shed much light on how premature birth affects the early activity of neurons in the frontal lobe, significant specifically to cognitive functions.
Anton Tokariev, Susanna Stjerna, J Matias Palva, Sampsa Vanhatalo. Preterm Birth Changes Networks of Newborn Cortical Activity. Cerebral Cortex, 2018; DOI: 10.1093/cercor/bhy012
The figure depicts frontal networks of cortical interactions found in the present study. These networks were disclosed by computer analysis based on newborn brainwaves (electroencephalograph) shown in the background.Credit: Anton Tokariev
Post link
“It’s rude to point” - maybe not…
The old adage that ‘it’s rude to point’ might need a rethink after new research showed that young children struggle to make sense of common symbols like arrows, and respond best to a pointing finger to direct their gaze.
Psychologists from the University of Lincoln, UK, found that the attention of pre-school and early school year children is strongly influenced by the direction of a pointing finger – but other visual directional cues such as arrows or pictures of peeking eyes are often ineffective.
The new findings challenge previous theories that the ability to direct visual attention to where others are looking relies on an innate brain module. The findings indicate that this cognitive ability must develop with age.
Researchers asked 137 children aged between three and ten years to play a specially-designed computer game. In the game, the children were asked to keep their eyes on a cartoon character, Buzzy Bee, which repeatedly jumped unpredictably from the middle to the left or right of the computer screen.
The researchers used eye-tracking technology to study the children’s responses. The children were instructed to follow the character with their eyes, but not to pay attention to pictures of eyes, arrows and pointing fingers which also flashed up on screen. These ‘directional cues’ could be pointing in either the same direction as the bee or in another direction. The bee would then jump around the screen, and the researchers monitored where the child’s gaze followed, as well as how quickly and accurately they followed the bee when it was displayed with the visual cues.
The findings showed that the youngest age group – three to five year-olds – were only influenced by the pointing fingers and tended to look in the direction it was pointing. Older children demonstrated a stronger ability to follow the bee correctly despite the directional cues.
Researcher Professor Tim Hodgson, neuroscientist and Head of the University of Lincoln’s School of Psychology, said: “In infancy a child develops brain circuits in the frontal cortex of the brain, an area which allows us to control our eyes and attention.
“Children have to learn to link what they see in the world around them with the direction of interesting information and events. One of the first ’cues’ to attention that young children learn may be the direction of an adult’s pointing index finger.”
The experiment was carried out at the University of Lincoln’s annual Summer Scientist event, in which hundreds of children and their parents participate in a range of fun games, quizzes and challenges which inform real psychology research.
The study is published in the Springer academic journal Experimental Brain Research.
Post link

Right light on the mother’s belly may be important to the foetus
There may be a link between exposure to light during pregnancy and foetal brain development. New findings by researchers at Umeå University, Sweden, working in collaboration with American colleagues, may provide better understanding of certain neurological diseases later in life.
“Ultimately, this discovery may open up possibilities for using the right kind of light stimulation during pregnancy to reduce the risk of neurological disorders in adulthood,” says Professor Lena Gunhaga at Umeå Centre for Molecular Medicine, Umeå University.
The research group at Umeå University, together with researchers in the group of Professor Richard Lang in Cincinnati, USA, now demonstrate that a light receptor called Opsin 3 is already expressed in parts of the central and peripheral nervous systems during the early stages of foetal development. The Opsin 3 molecule has a broad but distinct expression that suggests an important role in the formation of various neurons, neural pathways and areas of the brain and spinal cord. Opsin 3 expression can be linked to a number of motor and sensory neural pathways that regulate movement, pain, vision and olfaction, as well as memory, mood and emotion.
While the idea that light may affect cells inside the body, even in the unborn foetus, may seem peculiar, both calculations and experiments have previously shown that light can pass through skin, soft tissue and the skull to activate photoreceptors.
Opsin 3 detects light in the blue range at a wavelength of approximately 480 nanometres. The Umeå researchers’ discovery of the expression pattern of this receptor suggests that light plays a vital role in the development and subsequent function of the brain. This might explain why the risk of certain neurological and psychiatric diseases varies depending on the seasonal time of birth. So far, this unexplained correlation have been observed in diseases such as Parkinson’s, Alzheimer’s, multiple sclerosis, bipolar disorder, autism, schizophrenia and epilepsy. That said, time of birth is only one of several risk factors for the diseases in question.
“Although more research is required before we can issue recommendations about specific light therapies for pregnant women, we are clearly on an exciting track that may eventually prove highly significant,” says Lena Gunhaga.
While the new findings are based on observations of the brain and nervous system of mice, the function is deemed to be similar in humans. The researchers continue with more detailed studies of how Opsin 3 affects the development and function of the brain. The study is published in the scientific journal eNeuro.
(Image caption: A 3D image of an early mouse fetus showing initial Opsin3 expression in red against a background of anatomical structures in blue. The image was taken with optical project tomography. Credit: Wayne Davies)

(Image caption: Anterior neural folds of a mouse embryo imaged at Advanced Light Microscopy technology platform at MDC. Credit: Hammes Lab, MDC)
Disease genes help early brain development
If the cerebral hemispheres of the forebrain fail to divide properly in an unborn child, this results in holoprosencephaly. An MDC team led by Annette Hammes has discovered candidate genes that can positively influence the severity of this congenital malformation of the forebrain, as the researchers now report in the journal Development.
Holoprosencephaly (HPE) affects around one to four in every 1,000 unborn babies, and occurs when the cerebral hemispheres of the forebrain fail to divide or only partially divide. It is the most common malformation of the forebrain in humans and is associated with facial disfigurements, such as cleft lip and cleft palate or eyes that are very close together – even to the extent that the two eyeballs merge. The majority of fetuses affected die while still in the womb.
The exact causes of HPE are not yet fully understood. In addition to environmental pollutants and illness of the expectant mother, genetic factors can play a role – including mutations in the genes of the so-called Sonic Hedgehog (SHH) signaling pathway. This pathway controls the embryonic development of organs and the nervous system. Gene defects and a resulting loss of function of LRP2, an SHH co-receptor, result in brain defects that manifest themselves very differently. “We wanted to know why the severity of this disease varies so much,” says Dr. Annette Hammes, head of the Molecular Pathways in Cortical Development Lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). “While some sufferers have no or only mild symptoms, others have to live with severe deformities – even if the two patients are related to each other and we can therefore assume that the disease is caused by the same gene mutation.”
Disease genes restore the SHH pathway
Researchers have long assumed that there are genes that positively influence this malformation or even prevent it altogether. The Hammes Lab team has now identified two new candidates: “ULK4 and PTTG1, also known as securin,” says co-lead author Dr. Nora Mecklenburg, who was a postdoctoral researcher with Hammes at the time of the study. ULK4 is a gene that has so far been associated with schizophrenia and bipolar disorder, while PTTG1 is mainly researched in connection with cancer. In the journal Development, the scientists explain how these proteins can restore an impaired SHH pathway. Nine years of work went into the study, which the journal classifies as a “research highlight.”
But the results were also partly down to chance. Hammes and her team had been studying mice with LRP2 mutations for many years, together with the MDC lab led by Professor Thomas Willnow. “We know that LRP2 influences the formation of the neural tube in early embryogenesis, and it is out of this that the nervous system later develops,” explains the neuroscientist. Without LRP2, the SHH pathway is not sufficiently activated and malformations of the neural tube occur at a very early stage of pregnancy – often resulting in miscarriage. When the scientists crossed the LRP2 mutants of the commonly used black-coated mouse strain (known as “Black 6” for short) with another mouse strain with white coats, the result was very surprising: The white-coated offspring did not show any malformations of the brain or face – despite having a mutation in the SHH co-receptor LRP2. The team concluded that there must be as-yet-unknown factors that influence the SHH pathway, and set out to find them.
RNA analysis with high-throughput sequencing
To do this, Mecklenburg first bred the different mouse strains and examined the animals with regard to their disease characteristics, signaling pathways and genetic makeup. Together with her co-lead authors Franziska Witte, then a doctoral student in Professor Norbert Hübner’s MDC lab, and Izabela Kowalczyk, a doctoral student with Hammes, she sequenced and analyzed the RNA of embryonic cells of the different strains using high-throughput methods. The three scientists discovered that, despite having a similar genome sequence, the cells have completely different transcriptomes – meaning the genes are read very differently. “The extent of the differences even between the wild types of the two mouse strains at this early stage of embryonic development really surprised us,” says Mecklenburg.
The researchers conducted further studies and found that, in the white-coated LRP2 mutants, as well as in the wild types of this strain, certain genes, including ULK4 and PTTG1, are strongly upregulated compared to the Black 6 mice. To see if this affects the SHH pathway, they introduced the genes into cells lacking LRP2 function. “We were able to see that they significantly boost the Sonic Hedgehog signaling pathway,” says Kowalczyk. Their conclusion: “More ULK4 and PTTG1 are produced in the LRP2 mutants with the white mouse ancestors. They compensate for the missing LRP2, restore a sufficiently strong SHH pathway, and prevent the malformations from occurring.” This finding casts the disease genes ULK4 and PTTG1 in a completely new light: While high levels of expression can trigger diseases in the adult organism, they can actually positively influence the development of an embryo. The scientists were also able to pinpoint the location from which these factors amplify the SHH pathway – the antenna-like projections of the neuroepithelial cells, which are the cells that line the inside of the neural tube.
Decoding - and maybe even preventing - genetic diseases
“The fact that we have identified these candidate genes that modulate the SHH pathway in mice takes our knowledge of holoprosencephaly and other genetic diseases one step further,” says Hammes. “With this knowledge, we may even find a way to prevent them.” But there is still a long way to go until then, and the next step for her team is to explore what role the newly discovered SHH pathway modifiers play – not only during embryonic development, but also in the adult brain. The scientists have already located PTTG1 in the cytoskeleton of neurons – a protein network in the cytoplasm that gives the cells stability. The team is currently investigating what role the gene plays in this location.

The National Institutes of Health (NIH) has awarded researchers at University of California San Diego approximately $30 million over five years to expand and deepen longitudinal studies of the developing brain in children.
“This is a groundbreaking study of normal and atypical brain developmental trajectories from day 0 to 10 years of age in a large sample of about 8,000 families,” Christina Chambers, PhD, MPH, professor of pediatrics at UC San Diego School of Medicine and professor in the Herbert Wertheim School of Public Health and Human Longevity Science at UC San Diego.