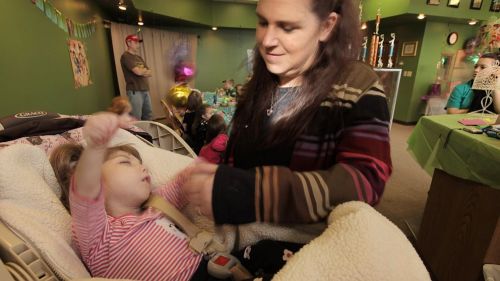#genetics
8-Year-Old Never Ages, Could Reveal ‘Biological Immortality’
Gabby Williams has the facial features and skin of a newborn, and she is just as dependent. Her mother feeds, diapers and cradles her tiny frame as she did the day she was born.
The little girl from Billings, Mont., is 8 years old, but weighs only 11 pounds. Gabby has a mysterious condition, shared by only a handful of others in the world, that slows her rate of aging.
For the past two years, a doctor who has been trying to find the genetic off-switch to stop the aging process has been studying Gabby, as well as two other people who have striking similarities.
Why the 'Benjamin Button’ children never age.
A 29-year-old Florida man has the body of a 10-year-old, and a 31-year-old Brazilian woman is the size of a 2-year-old. Like Gabby, neither seems to grow older.
Unraveling what these three people may have in common is the subject of a TLC television special, “40-Year-Old Child: A New Case,” which airs Monday, Aug. 19, at 10 p.m. ET. The show is a follow-up to Gabby’s story, which aired last year.
“In some people, something happens to them and the development process is retarded,” said medical researcher Richard F. Walker. “The rate of change in the body slows and is negligible.”
16-year-old is the size of a toddler.
Walker is retired from the University of Florida Medical School and now does his research at All Children’s Hospital in St. Petersburg.
“My whole career has been focused on the aging process,” he told ABCNews.com. “My fixation has been not on the consequences but the cause of it.”
Not only do the people he’s studying have a growth rate of one-fifth the speed of others, but they live with a variety of other medical problems, including deafness, the inability to walk, eat or even speak.
“Gabrielle hasn’t changed since pretty much forever,” said her mother, Mary Margret Williams, 38. “She has gotten a little longer and we have jumped into putting her in size 3-6 month clothes instead of 0-3 months for the footies.
"Last time we weighed her she was up a pound to 11 pounds and she’s gotten a few more haircuts,” she told ABCNews.com. “Other than that, she hasn’t changed much since the [2012] show.”
Williams, who works part-time at a dermatologist’s office, and her husband, a corrections officer for the state, share the child care responsibilities for their perpetual infant.
Walker explains that physiological change, or what he calls “developmental inertia,” is essential for human growth. Maturation occurs after reproduction.
“Without that process we never develop,” he said. “When we develop, all the pieces of our body come together and change and are coordinated. Otherwise, there would be chaos.”
But, said Walker, the body does not have a “stop switch” for this development. “What happens is we become mature at age 20 and continue to change.”
The first subtle internal body changes of aging are seen in the 30s and become more visible in the 40s.
“There is a progressive erosion of internal order as a result of developmental inertia,” he said.
In one of the girls Walker has studied, he found damage to one of the genes that causes developmental inertia, a finding that he said is significant. He also suspects the mutations are on the regulatory genes on the second female X chromosome.
“If we could identify the gene and then at young adulthood we could silence the expression of developmental inertia, find an off-switch, when you do that, there is perfect homeostasis and you are biologically immortal.”
Now Walker doesn’t mean that people will never die. Disease and accidents will still end human life.
“But you wouldn’t have the later years – you’d remain physically and functionally able,” he said.
That is why he believes his study of Gabby Williams’ genetic code is so important. “She fits the model,” said Walker.
“We’ve been on this journey to find out, are my other children at any risk in having a child like Gabrielle,” said Williams, who has five other children between the ages of 1 and 10.
“We did find out with Dr. Walker when he did the [gene] sequencing that it’s not something we can pass on but just an abnormality, a mutated gene that was just happenstance,” she said. “That was a relief for us.”
At first, when the Williams family members found out about Walker’s research, they hesitated to become guinea pigs in the studies that would promote a so-called “fountain of youth.”
“There was some concern,” she said. “We are good Catholics, God-fearing people and we believe we are meant to get old – the process of life – and meant to die. It was scary to think about, and we did not want to be part of it.”
But as they talked further with Walker, the family realized that his research was designed to help people struggling with the impairments of old age.
“Alzheimer’s is one of the scariest diseases out there,” said Williams. “If what Gabrielle holds inside of her would find a cure – for sure we would be a part of the research project. We have faith that Dr. Walker and the scientific community do find something focused more on the disease of aging, rather than making you 35 for the rest of your life.”
As for Gabby’s life span, her doctors cannot say what that will look like.
“From the time of her birth, we didn’t think she would be with us very long,” said her mother. “The fact is she is now going on 9 years. She kind of surpassed my expectations from the get go.
"It’s not something I worry about,” said Williams, who said she trusts that God has a plan for her infantile daughter.
“When he is ready to take her back, it will be sad,” she said. “But what a glorious thing it will be for Gabby to go to heaven one day. I know it will happen, but I am not hoping it’s any day soon.”
Post link
The cells in our bodies can divide as often as once every 24 hours, creating a new, identical copy. DNA binding proteins called transcription factors are required for maintaining cell identity. They ensure that daughter cells have the same function as their mother cell, so that for example muscle cells can contract or pancreatic cells can produce insulin. However, each time a cell divides the specific binding pattern of the transcription factors is erased and has to be restored in both mother and daughter cells. Previously it was unknown how this process works, but now scientists at Karolinska Institutet have discovered the importance of particular protein rings encircling the DNA and how these function as the cell’s memory.
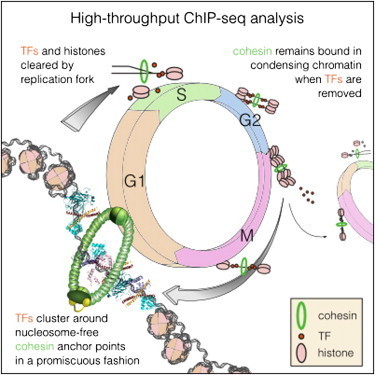
The DNA in human cells is translated into a multitude of proteins required for a cell to function. When, where and how proteins are expressed is determined by regulatory DNA sequences and a group of proteins, known as transcription factors, that bind to these DNA sequences. Each cell type can be distinguished based on its transcription factors, and a cell can in certain cases be directly converted from one type to another, simply by changing the expression of one or more transcription factors. It is critical that the pattern of transcription factor binding in the genome be maintained. During each cell division, the transcription factors are removed from DNA and must find their way back to the right spot after the cell has divided. Despite many years of intense research, no general mechanism has been discovered which would explain how this is achieved.
“The problem is that there is so much DNA in a cell that it would be impossible for the transcription factors to find their way back within a reasonable time frame. But now we have found a possible mechanism for how this cellular memory works, and how it helps the cell remember the order that existed before the cell divided, helping the transcription factors find their correct places”, explains Jussi Taipale, professor at Karolinska Institutet and the University of Helsinki, and head of the research team behind the discovery.
The results are now being published in the scientific journal Cell. The research group has produced the most complete map yet of transcription factors in a cell. They found that a large protein complex called cohesin is positioned as a ring around the two DNA strands that are formed when a cell divides, marking virtually all the places on the DNA where transcription factors were bound. Cohesin encircles the DNA strand as a ring does around a piece of string, and the protein complexes that replicate DNA can pass through the ring without displacing it. Since the two new DNA strands are caught in the ring, only one cohesin is needed to mark the two, thereby helping the transcription factors to find their original binding region on both DNA strands.
“More research is needed before we can be sure, but so far all experiments support our model,” says Martin Enge, assistant professor at Karolinska Institutet.
Transcription factors play a pivotal role in many illnesses, including cancer as well as many hereditary diseases. The discovery that virtually all regulatory DNA sequences bind to cohesin may also end up having more direct consequences for patients with cancer or hereditary diseases. Cohesin would function as an indicator of which DNA sequences might contain disease-causing mutations.
“Currently we analyse DNA sequences that are directly located in genes, which constitute about three per cent of the genome. However, most mutations that have been shown to cause cancer are located outside of genes. We cannot analyse these in a reliable manner - the genome is simply too large. By only analysing DNA sequences that bind to cohesin, roughly one per cent of the genome, it would allow us to analyse an individual’s mutations and make it much easier to conduct studies to identify novel harmful mutations,” Martin Enge concludes.
The active ingredient in an over-the-counter skin cream might do more than prevent wrinkles. Scientists have discovered that the drug, called kinetin, also slows or stops the effects of Parkinson’s disease on brain cells.
Scientists identified the link through biochemical and cellular studies, but the research team is now testing the drug in animal models of Parkinson’s. The research is published in the August 15, 2013 issue of the journal Cell.
“Kinetin is a great molecule to pursue because it’s already sold in drugstores as a topical anti-wrinkle cream,” says HHMI investigator Kevan Shokat of the University of California, San Francisco. “So it’s a drug we know has been in people and is safe.”
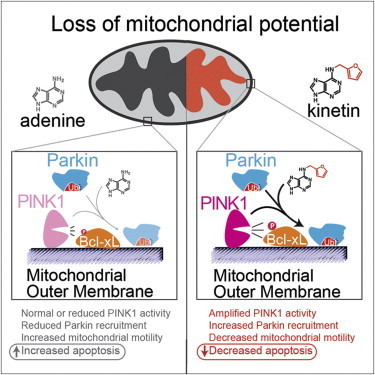
Parkinson’s disease is a degenerative disease that causes the death of neurons in the brain. Initially, the disease affects one’s movement and causes tremors, difficulty walking, and slurred speech. Later stages of the disease can cause dementia and broader health problems. In 2004, researchers studying an Italian family with a high prevalence of early-onset Parkinson’s disease discovered mutations in a protein called PINK1 associated with the inherited form of the disease.
Since then, studies have shown that PINK1 normally wedges into the membrane of damaged mitochondria inside cells that causes another protein, Parkin, to be recruited to the mitochondria, which are organelles responsible for energy generation. Neurons require high levels of energy production, therefore when mitochondrial damage occurs, it can lead to neuronal death. However, when Parkin is present on damaged mitochondria, studding the mitochondrial surface, the cell is able to survive the damage. In people who inherit mutations in PINK1, however, Parkin is never recruited to the organelles, leading to more frequent neuronal death than usual.
Shokat and his colleagues wanted to develop a way to turn on or crank up PINK1 activity, therefore preventing an excess of cell death, in those with inherited Parkinson’s disease. But turning on activity of a mutant enzyme is typically more difficult than blocking activity of an overactive version.
“When we started this project, we really thought that there would be no conceivable way to make something that directly turns on the enzyme,” says Shokat. “For any enzyme we know that causes a disease, we have ways to make inhibitors but no real ways to turn up activity.”
His team expected it would have to find a less direct way to mimic the activity of PINK1 and recruit Parkin. In the hopes of more fully understanding how PINK1 works, they began investigating how PINK1 binds to ATP, the energy molecule that normally turns it on. In one test, instead of adding ATP to the enzymes, they added different ATP analogues, versions of ATP with altered chemical groups that slightly change its shape. Scientists typically must engineer new versions of proteins to be able to accept these analogs, since they don’t fit into the typical ATP binding site. But to Shokat’s surprise, one of the analogs—kinetin triphosphate, or KTP—turned on the activity of not only normal PINK1, but also the mutated version, which doesn’t bind ATP.
“This drug does something that chemically we just never thought was possible,” says Shokat. “But it goes to show that if you find the right key for the right lock, you’ll be able to open the door.”
To test whether the binding of KTP to PINK1 led to the same consequences as the usual ATP binding, Shokat’s group measured the activity of PINK1 directly, as well as the downstream consequences of this activity, including the amount of Parkin recruited to the mitochondrial surface, and the levels of cell death. Adding the precursor of KTP, kinetin, to cells—both those with PINK1 mutations and those with normal physiology—amplified the activity of PINK1, increased the level of Parkin on damaged mitochondria, and decreased levels of neuron death, they found.
“What we have here is a case where the molecular target has been shown to be important to Parkinson’s in human genetic studies,” says Shokat. “And now we have a drug that specifically acts on this target and reverses the cellular causes of the disease.”
The similar results in cells with and without PINK1 mutations suggest that kinetin, which is a precursor to KTP, could be used to treat not only Parkinson’s patients with a known PINK1 mutation, but to slow progression of the disease in those without a family history by decreasing cell death.
Shokat is now performing experiments on the effects of kinetin in mice with various forms of Parkinson’s disease. However, the usefulness of animal models in Parkinson’s research has been debated, and therefore the positive results from the cellular data, he says, is as good an indicator as results in animals that this drug has potential to treat Parkinson’s in humans. Initial human studies will likely focus on the small population of patients with PINK1 mutations, and if successful in that group the drug could later be tested in a wider array of Parkinson’s patients.
A Genetic Answer to the Alzheimer’s Riddle?
What if we could pinpoint a hereditary cause for Alzheimer’s, and intervene to reduce the risk of the disease? We may be closer to that goal, thanks to a team at the University of Kentucky. Researchers affiliated with the UK Sanders-Brown Center on Aging have completed new work in Alzheimer’s genetics; the research is detailed in a paper published today in the Journal of Neuroscience.
Emerging evidence indicates that, much like in the case of high cholesterol, some Alzheimer’s disease risk is inherited while the remainder is environmental. Family and twin studies suggest that about 70 percent of total Alzheimer’s risk is hereditary.
Recently published studies identified several variations in DNA sequence that each modify Alzheimer’s risk. In their work, the UK researchers investigated how one of these sequence variations may act. They found that a “protective” genetic variation near a gene called CD33 correlated strongly with how the CD33 mRNA was assembled in the human brain. The authors found that a form of CD33 that lacked a critical functional domain correlates with reduced risk of Alzheimers disease. CD33 is thought to inhibit clearance of amyloid beta, a hallmark of Alzheimers disease.
The results obtained by the UK scientists indicate that inhibiting CD33 may reduce Alzheimer’s risk. A drug tested for acute myeloid leukemia targets CD33, suggesting the potential for treatments based on CD33 to mitigate the risk for Alzheimer’s disease. Additional studies must be conducted before this treatment approach could be tested in humans.
Post link
How A Pregnant Woman’s Choices Could Shape A Child’s Health
Pregnant women hear a lot about things they should avoid: alcohol, tobacco, chemical exposures, stress. All of those have the potential to affect a developing fetus. And now scientists are beginning to understand why.
One important factor, they say, is something called epigenetics, which involves the mechanisms that turn individual genes on and off in a cell.
There’s growing evidence that epigenetics is critical in determining a child’s risk of developing problems ranging from autism to diabetes, says Dani Fallin, who studies the genetics of mental disorders at Johns Hopkins Bloomberg School of Public Health.
Epigenetic control of genes is part of what allows a tiny cluster of identical cells in the womb to grow into a fully formed baby. By switching certain genes on and off, some cells become heart cells while others become brain cells.
It’s a delicate process that can be disrupted by exposure to certain chemicals or hormones, says Susan Kay Murphy, an associate professor of obstetrics and gynecology at Duke University School of Medicine. And the first week or so after conception appears to be “a particularly vulnerable time where environmental influences can directly affect an epigenetic outcome,” she says.
Murphy’s interest in epigenetics is personal as well as professional. She entered the field in the 1990s after her young son died from a rare form of liver cancer that has been linked to epigenetic changes. She also has a son with autism and a daughter with ADHD.
Illustration by Katherine Streeter for NPR
Post link
This is an elaboration of this post.gilivhan does a pretty good job of explaining things, but I noticed an error in generation two (regarding the law of independent assortment), so, as a biology major, I thought I could help with this.
I’m warning you, do not venture forth lightly. This is a verylong post.
Physical attraction is linked to heightgenes
Scientists have discovered that who you find attractive is partly down to the genes affecting your height.
In a study of more than 13,000 heterosexual couples they found that 89% of the genetic variation that controls a person’s height also influences their height preference in a partner.
Generally people are attracted to partners of similar heights to themselves.
Did you know that our choice in partners can have important consequences for human populations? This study brings us closer to understanding the complexity of sexual attraction and the mechanisms that drive variation in humans.
Images: Susana Fernandez, Boris SV
Post link
Space sleep study to understand ageing
Tomorrow the first official British astronaut Tim Peake will blast into space for 6 months.
He’ll be doing lots of experiments and tests that aren’t possible on Earth because of the unique conditions on the ISS 400km above Earth. He’ll even be running the London Marathon from space.
But living in microgravity for so long will take a toll on his body; astronauts experience bone and muscle loss, diminished immune systems and increased inflammation, complaints that also are common in the elderly.
To help find out why this happens an experiment at the University of Surrey with the European Space Agency is mimicking the effects of microgravity. Young healthy men will spend two weeks living in the lab ‘normally’ before spending 60 days constantly in beds that are slightly tilted to simulate the microgravity on board the ISS.
By investigating how these conditions disrupt sleep and body clocks, the study will help figure out the genetic processes that contribute to the health problems experienced by both the elderly and astronauts in space.
Photos: NASA and Victor Zelentsov
Post link
How workers can become queens
A honey bee’s fate is decided at birth. The larvae develop to become a queen or a worker. If you’re born a queen, you get to rule the hive.
But other insects are more flexible.
For example, paper wasps and dinosaur ants are able to switch role from worker to queen at any point in their life - and new research uncovers the basis of this flexibility.
Researchers from the University of Bristol, the Babraham Institute and the Centre for Genomic Regulation analysed individual wasp and ant brains from queens and workers of both species to see whether caste differences could be explained by variations in how the genome is ‘read’ and regulated.In the paper wasps as seen in the video above, the queen is identifiable by behaviours such as shaking the abdomen and aggression to exert dominance.
By looking at the genetic makeup of the insects, the researchers were able to determine what genetic influences were controlling behaviour.
They found very little difference between roles, which was surprising given that hundreds of genes are involved in determining the differences between queens and workers in the honeybee.
This suggests that there is no single master gene determining the role of these wasps and ants.
So you don’t have to be born a queen after all…
Video: Solenn Patalano
How the world’s first plants took a giant leap on to land
About 450M years ago, plants in the sea took a giant leap on to land to become the plants we know and love today. But how did they survive?
Scientists for The John Innes Centre may have found the answer.
Land plants survive by getting water and nutrients from soil.
They do this by forming a special friendship with soil dwelling fungi called mycorrhiza. These strands of fungi reach deep into the spoil and drag the nutrients and water back to the plants.
But when the first algae landed on soil, how did they survive long enough to form these beneficial friendships?
New research suggests that they already had the genes necessary for forming this bond whilst they lived in the sea.
Researchers analyzed DNA and RNA of some of the earliest known land plants and green algae and found evidence that their shared algal ancestors living in the Earth’s waters already possessed the necessary set of genes needed to detect and interact with the beneficial fungi.
The team of scientists believes this capability was pivotal in enabling the alga to survive out of the water and to colonise the earth. By working with the fungi to find sustenance, the alga had an evolutionary advantage and could thrive in a very different and seemingly infertile environment.
This was a watershed moment that kick-started the evolution of life on earth.
Be part of an important study on the genetics of sexual orientation
Have you had your DNA analyzed by 23andMe or Ancestry?
Are you 18 years or older?
If you answered YES to these questions, you are eligible to participate in a study on sexual orientation.
The purpose of this research study is to understand how genetics may influence people’s personalities and sexual orientation. If you take part in this online study, we will instruct you how to find your genetic data file on your 23andMe account and upload it to our secure website. We will also ask you to complete a series of questionnaires on your personality and sexual behavior.
Time required to complete the study should be about 15-25 minutes.
Anyone 18 years or older who has been sexually active and has had a 23andMe or Ancestry analysis is eligible to participate, regardless of sexual orientation.
Please follow this link to begin the study:
https://pennstate.qualtrics.com/SE/?SID=SV_e5Vi2kF7dFeGGr3
This study is being conducted by the Department of Anthropology at Penn State University, 409 Carpenter Building, University Park, PA.
Please contact the study coordinator Heather Self ([email protected]) or the principal investigator David Puts ([email protected]) for further information.
Be part of an important study on the genetics of sexual orientation
· Have you had your DNA analyzed by 23andMe or Ancestry?
· Are you 18 years or older?
If you answered YES to these questions, you are eligible to participate in a study on sexual orientation.
The purpose of this research study is to understand how genetics may influence people’s personalities and sexual orientation. If you take part in this online study, we will instruct you how to find your genetic data file on your 23andMe account and upload it to our secure website. We will also ask you to complete a series of questionnaires on your personality and sexual behavior.
Time required to complete the study should be about 15-25 minutes.
Anyone 18 years or older who has been sexually active and has had a 23andMe or Ancestry analysis is eligible to participate, regardless of sexual orientation.
Please follow this link to begin the study:
https://pennstate.qualtrics.com/SE/?SID=SV_e5Vi2kF7dFeGGr3
This study is being conducted by the Department of Anthropology at Penn State University, 409 Carpenter Building, University Park, PA.
Please contact the study coordinator Heather Self ([email protected]) or the principal investigator David Puts ([email protected]) for further information.
Be part of an important study on the genetics of sexual orientation
· Have you had your DNA analyzed by 23andMe or Ancestry?
· Are you 18 years or older?
If you answered YES to these questions, you are eligible to participate in a study on sexual orientation.
The purpose of this research study is to understand how genetics may influence people’s personalities and sexual orientation. If you take part in this online study, we will instruct you how to find your genetic data file on your 23andMe account and upload it to our secure website. We will also ask you to complete a series of questionnaires on your personality and sexual behavior.
Time required to complete the study should be about 15-25 minutes.
Anyone 18 years or older who has been sexually active and has had a 23andMe or Ancestry analysis is eligible to participate, regardless of sexual orientation.
Please follow this link to begin the study:
https://pennstate.qualtrics.com/SE/?SID=SV_e5Vi2kF7dFeGGr3
This study is being conducted by the Department of Anthropology at Penn State University, 409 Carpenter Building, University Park, PA.
Please contact the study coordinator Heather Self ([email protected]) or the principal investigator David Puts ([email protected]) for further information.
Be part of an important study on the genetics of sexual orientation
· Have you had your DNA analyzed by 23andMe or Ancestry?
· Are you 18 years or older?
If you answered YES to these questions, you are eligible to participate in a study on sexual orientation.
The purpose of this research study is to understand how genetics may influence people’s personalities and sexual orientation. If you take part in this online study, we will instruct you how to find your genetic data file on your 23andMe account and upload it to our secure website. We will also ask you to complete a series of questionnaires on your personality and sexual behavior.
Time required to complete the study should be about 15-25 minutes.
Anyone 18 years or older who has been sexually active and has had a 23andMe or Ancestry analysis is eligible to participate, regardless of sexual orientation.
Please follow this link to begin the study:
https://pennstate.qualtrics.com/SE/?SID=SV_e5Vi2kF7dFeGGr3
This study is being conducted by the Department of Anthropology at Penn State University, 409 Carpenter Building, University Park, PA.
Please contact the study coordinator Heather Self ([email protected]) or the principal investigator David Puts ([email protected]) for further information.
Be part of an important study on the genetics of sexual orientation
· Have you had your DNA analyzed by 23andMe or Ancestry?
· Are you 18 years or older?
If you answered YES to these questions, you are eligible to participate in a study on sexual orientation.
The purpose of this research study is to understand how genetics may influence people’s personalities and sexual orientation. If you take part in this online study, we will instruct you how to find your genetic data file on your 23andMe account and upload it to our secure website. We will also ask you to complete a series of questionnaires on your personality and sexual behavior.
Time required to complete the study should be about 15-25 minutes.
Anyone 18 years or older who has been sexually active and has had a 23andMe or Ancestry analysis is eligible to participate, regardless of sexual orientation.
Please follow this link to begin the study:
https://pennstate.qualtrics.com/SE/?SID=SV_e5Vi2kF7dFeGGr3
This study is being conducted by the Department of Anthropology at Penn State University, 409 Carpenter Building, University Park, PA.
Please contact the study coordinator Heather Self ([email protected]) or the principal investigator David Puts ([email protected]) for further information.
Bolivar Trask makes his first appearance in the movie
Me: Achondroplasia!
A: He’s a great actor
Me: I know, but I hope you remember what gene it’s on.
A: Oh gosh, stop it.
Trask mentions the Mutant X gene
Me: I wonder if that’s on the X chromosome?
A: Hmm.
Me: I should research this. Maybe I’ll write a paper on the genetics of all this.
Where to Draw the Line on Gene-Editing Technology
The biologists have done it again. Not so long ago it was cloning and embryonic stem cells that challenged moral imagination. These days all eyes are on a powerful new technique for engineering or “editing” DNA. Relatively easy to learn and to use, CRISPR has forced scientists, ethicists and policymakers to reconsider one of the few seeming red lines in experimental biology: the difference between genetically modifying an individual’s somatic cells and engineering the germline that will be transmitted to future generations. Instead of genetic engineering for one person why not eliminate that disease trait from all of her or his descendants?
This week, the U.S. National Academy of Sciences, the Chinese Academy of Sciences, and the U.K. Royal Society are trying to find ways to redraw that red line. And redraw it in a way that allows the technology to help and not to hurt humanity. Perhaps the hardest but most critical part of the ethical challenge: doing that in a way that doesn’t go down a dark path of “improvements” to the human race.
Image credit: the Wilson Center
Post link