#hydrogen spectrum
** This note was inspired from a comment on one of our previous post. Do check it out before you read this one.
The emission spectrum of atomic hydrogen is given by this amazing spectral series diagram:

Let’s take a closer look at only the visible portion of the spectrum i.e the Balmer series.

If a hydrogen lamp and a diffraction grating just happen to be with you, you can take a look at the hydrogen lamp through the diffraction grating, these lines are what you would see:

These are known emission lines and they occur when the hydrogen atoms in the lamp return to a state of lower energy from an excited energy state.

Representation of emission and absorption using the Bohr’s model
Here’s another scenario that could also happen:

You have a bright source of light with a continuous spectrum and in between the source and the screen, you introduce a gas (here, sodium)
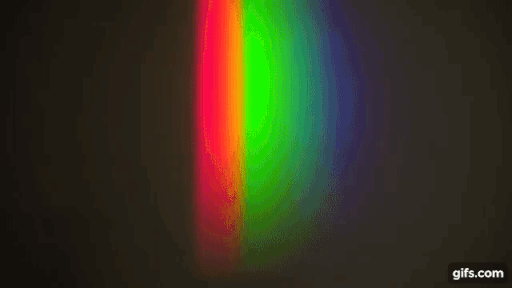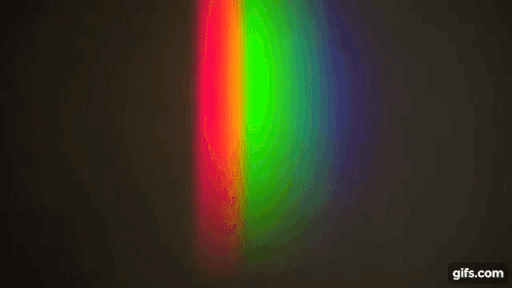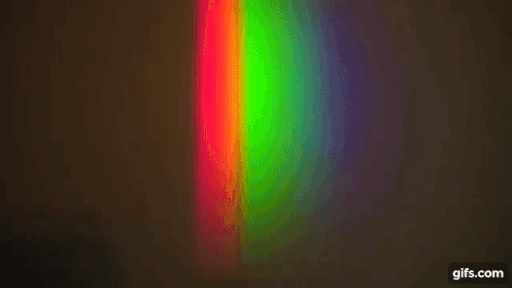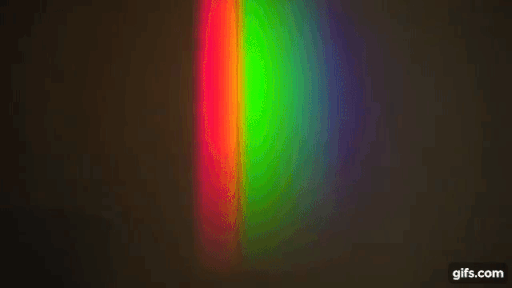
Source: Harvard Natural sciences
The gas absorbs light at particular frequencies and therefore we get dark lines in the spectrum.
This is known as absorption line. The following diagram summarizes what was told thus-far in a single image:
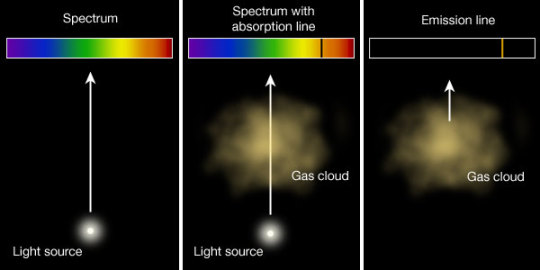
The absorption and emission spectrum for hydrogen look like so :

Stars and Hydrogen
One of the comments from the previous post was to show raw spectrum data of what was being presented to get a better visual aid.
Therefore,the following spectrum is a spectrum of a star taken from the Sloan Digital Sky Survey (SDSS)

Plot of wavelength vs median-flux
Here’s the spectrum with all the absorption lines labelled:
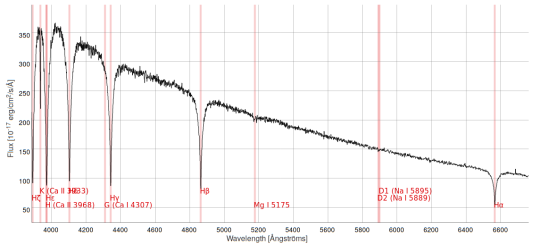
You can clearly see the Balmer series of hydrogen beautifully encoded in this spectrum that was taken from a star that is light-years away.
And astronomers learn to grow and love these lines and identify them immediately in any spectrum, for they give you crucial information about the nature of the star, its age, its composition and so much more.

Have a great day!
*If you squint your eyes a bit more you can find other absorption lines from other atoms embedded in the spectrum as well.
