#materialsposts
Alloys: 6061 Aluminum
Among the most popular aluminum alloys, 6061 aluminum is an alloy in the 6000 series of aluminum alloys: those heat treatable alloys where the principle alloying elements are magnesium and silicon. Because it is so popular, 6061 aluminum is also one of the least expensive of the aluminum alloys.
Highly resistant to corrosion, this alloy can be tempered a variety of different ways to achieve the desirable properties. Different tempers can alter the workability, weldability, and strength. T6 is one of the most common tempers (solution heat treated and artificially aged), but other tempers include O (annealed), T1 (cooled from elevated temperature shaping process and naturally aged), and T4 (solution heat-treated and naturally aged).
6061 aluminum is composed of over 95% aluminum with small or trace amounts of silicon, iron, copper, manganese, magnesium, chromium, zinc, and titanium added in. Magnesium is the largest alloying element, at up to 1.2% maximum, followed by silicon at 0.80% maximum. It is very often extruded but is also suitable for hot forging.
While not as high strength as some of the other aluminum alloys, 6061 is still highly versatile and used in a wide variety of applications: railway car components; boat or aircraft structures; other structural components such as bridges; pipes; wheels; cans; and SCUBA tanks.
Sources/Further Reading: (1) ( 2 - image 2 ) (3) (4) (5)
Post link
Polymers: Polyphenylene oxide
Though its technical name is poly(2,6-dimethylphenylene oxide) it is most commonly known as poly(phenylene oxide) or PPO. A condensation polymer, the polymerization of PPO produces water as a byproduct. It is a type of polyphenylene and is often grouped alongside polyphylene ethers as well.
PPO is one of many high-performance polymers known as engineering thermoplastics. These polymers have higher glass transition temperatures, making them temperature resistant. However, these polymers are often more crystalline, and therefore more brittle. In order to help with processing of PPO and to increase the toughness, almost all commercially available forms of the polymer are blended with polystyrene (such as high-impact polystyrene, or HIPS).
Applications of PPO (or, more commonly, blends of PPO and polystyrene) include those shown on the chart above. PPO is often used in applications where a polymer is desired but high heat resistance is also necessary, such as certain structural applications, electronics, and medical equipment (such as sterilizable instruments). Another common usage is in air separation membranes for the generation of nitrogen, an application which uses hollow fibers to create a membrane.
Sources/Further Reading: ( 1 - image 1 ) ( 2 - image 2 ) ( 3 - image 3 ) (4) (5)
Post link
Polymers: Polysulfones
A category of polymers technically defined as any polymer which contains a sulfonyl group, the term polysulfone is actually most often used in reference to polyarylethersulfones, in which the following structure is present: aryl-SO2-aryl. These polymers are thermoplastics, and known for their toughness and stability at high temperatures.
Polysulfones are amorphous polymers typically prepared through condensation polymerizations and are rigid and high-strength. They stand up well in high-pressure environments and are often considered to be high-performance polymers. These polymers are often semi-transparent and resistant to creep and deformation at high temperatures under continuous loads.
These polymers are fairly uncommon, due to the relatively high costs of production and the raw materials involved, and as such applications of polysulfones tend to be highly specialized. Because of their high service temperatures, they are sometimes used as flame retardants, or in medical applications requiring autoclave or steam sterilization. Another common application is in the form of membranes, with controllable poor sizes.
Sources/Further reading: ( 1 - image 1 ) ( 2 - image 2 ) (3) (4)
Post link
Alloys: Brass
An alloy of copper and zinc that has been known about since ancient times, brass is stronger and more durable than its base components, though not as strong as steel. It has stuck around for so long because it’s easy to work with, though the malleability depends upon the zinc content, and is used in decorations and applications where low friction is required.
Brass is a substitutional alloy, typically composed of around 67% copper and 33% zinc. Alloys with less zinc are occasionally called red brass while alloys with more zinc (above 45%) are known as white brasses (and are not commonly used industrially). The table below shows some of the common classifications.
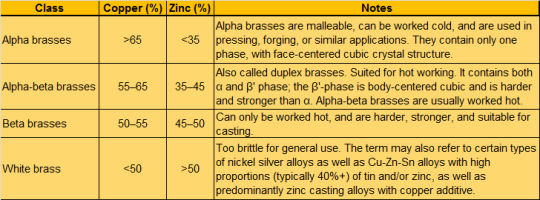
Other elements commonly added to brass include tin, iron, arsenic, antimony, aluminum and potentially small amounts of manganese, silicon, and phosphorus. Lead is also a common additive, though it is becoming less common thanks to growing concerns about its toxicity.
Aside from the properties mentioned above there are many more reasons why brass is a desirable metal. The high copper content makes brass anti-microbial, making it ideal for use in commonly touched items. Brass is also fairly corrosion resistant, allowing it to be used in plumbing. Instruments made of brass are also extremely common thanks to the alloys workability and durability.
Check out this link for a list of common brass alloys and this one for more in depth information on the properties of various alloys.
Image Sources: Top left,top right,middle left,middle right,bottom left,bottom right
Post link
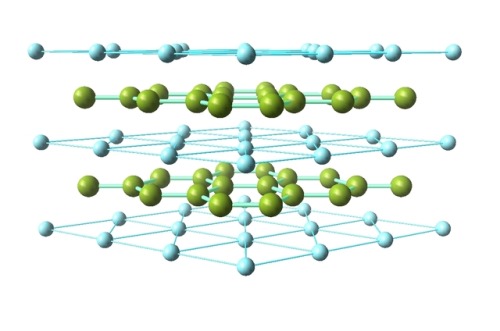
Ceramics: Magnesium diboride
Classified as a simple binary compound, magnesium diboride (MgB2) is also sometimes known as an intermetallic superconductor. It has a hexagonal crystal structure, which forms two-dimensional layers of boron in a graphite-like structure between the triangular layers of magnesium (as seen in the two images above).
MgB2‘s biggest claim to fame is its status as a relatively inexpensive superconductor. Among conventional superconductors (those that are phonon-mediated), it has one of the highest critical temperatures, at around 39 K. Though it had been known as a material to scientists for some time, its superconductivity was not discovered until 2001. Aside from being a high-temperature superconductor, MgB2 also has more than one superconducting energy gap, something theorized but rarely seen experimentally.
The bulk, polycrystalline form of the material can be easily made by exposing solid boron to magnesium vapor at high temperatures. Single crystals of the material are more difficult to form, but can be done so under high pressure. Thin films are often made through hybrid physical-chemical vapor deposition.
Applications (or possible applications) of MgB2 often take advantage of its superconductivity, such as in MRIs and tokamaks, but the material does have other suggested uses. The compound burns completely when ignited in oxygen, and so has been proposed for usage as a fuel in ramjets, or in blast-enhanced explosives and propellants.
Sources: ( 1 - image 3 ) (2) (3) (4) (5) (6)
Post link
Alloys: Steel
According to Dictionary.com, steel is “any of various modified forms of iron, artificially produced, having a carbon content less than that of pig iron and more than that of wrought iron, and having qualities of hardness, elasticity, and strength varying according to composition and heat treatment: generally categorized as having a high, medium, or low-carbon content”.
Perhaps the most well known alloy around, as well as one of the most common materials in the world, steel is essentially iron with a small percentage of carbon (and, on occasion, one or more other elements). Not enough carbon and you’re stuck with wrought iron, too much carbon and you get cast iron. The graph above is a binary iron-carbon phase diagram that goes from zero percent carbon to about 6.5 percent, illustrating the various phases that can form.
Steel has been known about since ancient times, some pieces dating back to 1800 BC, but it was the invention of the Bessemer process during the industrial revolution that really popularized the alloy. (Technically, similar methods had been used before, particularly in China and Japan, but Henry Bessemer invented the modern method, industrializing it and obtaining a patent in 1856.)
Mainly used in construction, the alloy has been used for almost every possible application: from office furniture to steel wool, from bulldozers to washing machines, and from wires to watches, the possibilities are pretty much endless. Steel is also one of the world’s most-recycled materials, able to be used more than once, with a recycling rate of over 60% globally.
The addition of carbon allows the steel to be stronger than the iron it’s made from. Adding nickel and manganese increases its tensile strength, chromium increases hardness and melting temperature, and vanadium also increases hardness while making it less prone to metal fatigue. Stainless steel has at least eleven percent chromium, whereas Hadfield steel (which resists wearing) contains twelve to fourteen percent manganese. Check out theselinks for more information on the effects of adding certain elements.
Sources:1 (top images),2 (bottom images)
Post link
Minerals: Wulfenite
A lead molybdenite mineral with the formula PbMoO4, wulfenite is typically found in the form of thin tabular crystals that range from orange-red to yellow-orange in color, though it can also be brown. It was named for Austrian mineralogist Franz Xaver Freiherr von Wulfen, though it wasn’t named until decades after his death.
Wulfenite crystallizes in the tetragonal crystal system. It is a secondary ore for both lead and molybdenum but, though it can be used to extract both of these elements, other, more commons ores are typically used. As such, wulfenite is usually only used by collectors.
For more in depth information about wulfenite, check out what mindat.org,webmineral.com,minerals.net, and handbookofmineralogy.org have to say about it.
Sources: (1) ( 2 - image 4 ) ( 3 - image 1 )
Image sources: ( 2 and 3 )
Post link