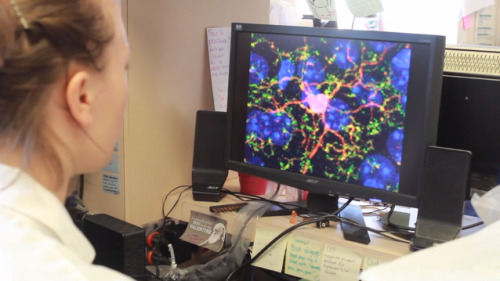
#microglia
Mechanism that reduces effect of cocaine on brain discovered
A type of brain cell known as microglia plays a key role in reducing the effects of cocaine in the brain, according to a major study by a team from the Research Institute of the McGill University Health Centre (RI-MUHC) in Montreal.
The discovery, published in the journal Neuron, establishes for the first time that microglia can diminish the adverse changes to neural circuitry brought on by the chronic use of cocaine and has significant implications for developing an effective treatment for addiction.
“What we discovered is that cocaine activates these microglia, which causes the release of an inflammatory signal which then tries to reverse the changes that cocaine is inducing in the neurons,” says the study’s senior author, Dr. David Stellwagen, a researcher from the Brain Repair and Integrative Neuroscience at the RI-MUHC and associate professor in the Department of Neurology and Neurosurgery at McGill University.
Microglia may not be as well known as neurons, the brain cells that relay messages, but they have many important functions. They constantly monitor their environment, and can act to maintain normal brain functioning. When they find something amiss, they can produce molecules that instruct neurons to make adaptive changes to their connections. One such example is the inflammatory molecule known as tumor necrosis factor (TNF).
Using a mouse model, researchers detected this microglia-mediated reversal by looking at how TNF acts on a particular set of synapses in the brain. “These connections are really important for regulating the behavior response in animal models to drugs of abuse such as cocaine,” says co-first author Sarah Konefal, a McGill PhD student in the Integrated Program in Neuroscience in Dr. Stellwagen’s lab.
The team found that TNF suppresses specific synaptic changes caused by cocaine–changes that are thought to underlie addiction. But Dr. Stellwagen explains that this beneficial mechanism doesn’t last. “The microglia response fades over time. One of the things that could transition somebody from just casual use into chronic dependency might be the fading of this adaptive signal which then allows the drugs to solidify their change to the neural circuitry.”
So can microglia be enticed to keep going? To find out, the team used a pharmaceutical agent that stimulates microglial production of TNF. Researchers observed that a cocaine-induced behavioral change in mice, the progressive increase in movement induced by cocaine,–was reduced in the animals who received this agent.
This exciting result holds promise for one day developing treatments that could cut down on drug relapse rates, which can run as high as 80 per cent. As Dr. Stellwagen puts it, “If we could develop a treatment that would suppress the craving that addicts have in stressful situations, or when they are re-exposed to situations in which they’d normally be taking the drug, that may allow them to avoid relapse. And that’s really the therapeutic goal of the work we have been doing.”
Dr. Stellwagen and colleagues are now investigating if the stimulated release of TNF can actually suppress cravings for cocaine. They are also hopeful that this work can be applied to other addictive substances including alcohol and methamphetamine.
Post link
In Neurodegenerative Diseases, Brain Immune Cells Have a “Ravenous Appetite” for Sugar
At the beginning of neurodegenerative disease, the immune cells of the brain – the “microglia” – take up glucose, a sugar molecule, to a much greater extent than hitherto assumed. Studies by the DZNE, the LMU München and the LMU Klinikum München, published in the journal “Science Translational Medicine”, come to this conclusion. These results are of great significance for the interpretation of brain scans depicting the distribution of glucose in the brain. Furthermore, such image-based data could potentially serve as a biomarker to non-invasively capture the response of microglia to therapeutic interventions in people with dementia.
In humans, the brain is one of the organs with the highest energy consumption, which can change with age and also due to disease – e. g. as a result of Alzheimer’s disease. “Energy metabolism can be recorded indirectly via the distribution of glucose in the brain. Glucose is an energy carrier. It is therefore assumed that where glucose accumulates in the brain, energy demand and consequently brain activity is particularly high,” says Dr. Matthias Brendel, deputy director of the Department of Nuclear Medicine at LMU Klinikum München.
The measuring technique commonly used for this purpose is a special variant of positron emission tomography (PET), known as “FDG-PET” in technical jargon. Examined individuals are administered an aqueous solution containing radioactive glucose that distributes in the brain. Radiation emitted by the sugar molecules is then measured by a scanner and visualized. “However, the spatial resolution is insufficient to determine in which cells the glucose accumulates. Ultimately, you get a mixed signal that stems not only from neurons, but also from microglia and other cell types found in the brain,” says Brendel.
Cellular Precision
“The textbook view is that the signal from FDG-PET comes mainly from neurons, because they are considered the largest consumers of energy in the brain,” says Christian Haass, research group leader at DZNE and professor of biochemistry at LMU Munich. “We wanted to put this concept to the test and found that the signal actually comes predominantly from the microglia. This applies at least in the early stages of neurodegenerative disease, when nerve damage is not yet so advanced. In this case, we see that the microglia take up large amounts of sugar. This appears to be necessary to allow them for an acute, highly energy-consuming immune response. This can be directed, for example, against disease-related protein aggregates. Only in the later course of the disease does the PET signal appear to be dominated by neurons.”
The findings of the Munich researchers are based on laboratory investigations as well as PET studies in about 30 patients with dementia – either Alzheimer’s disease or so-called four-repeat tauopathy. The findings are supported, for instance, by studies on mice whose microglia were either largely removed from the brain or, so to speak, deactivated. In addition, a newly developed technique was used that allowed cells derived from the brains of mice to be sorted according to cell type and their sugar uptake to be measured separately.
Consequences for Research and Practice
“FDG-PET is used in dementia research as well as in the context of clinical care,” Brendel says. “Insofar, our results are relevant for the correct interpretation of such brain images. They also shed new light on some hitherto puzzling observations. However, this does not call into question existing diagnoses. Rather, it is about a better understanding of the disease mechanisms.”
Haass draws further conclusions from the current results: “In recent years, it has become evident that microglia play a crucial, protective role in Alzheimer’s and other neurodegenerative diseases. It would be very helpful to be able to monitor the activity of these cells non-invasively, for example their response to drugs. In particular, to determine whether a therapy is working. Our findings suggest that this may be possible by PET.”

Immune cells in the brain share the work
To break down toxic proteins more quickly, immune cells in the brain can join together to form networks when needed. This is shown by a joint study of the University of Bonn, the German Center for Neurodegenerative Diseases (DZNE) and the Institut François Jacob in France. However, in certain mutations that can cause Parkinson’s disease, this cooperation is impaired. The findings are published in the renowned journal Cell.
The protein alpha-synuclein (abbreviated aSyn) performs important tasks in the nerve cells of the brain. But under certain circumstances, aSyn molecules can clump together and form insoluble aggregates. These damage the neurons; they are for instance typically found in the brains of people suffering from Parkinson’s disease or Lewy body dementia.
The immune cells of the brain, the microglial cells, therefore try to break down and dispose of the aSyn aggregates. This process is not only time-consuming; it can also cause the microglial cells themselves to perish. “We have now identified a mechanism that addresses both problems,” explains Prof. Dr. Michael Heneka. The researcher is director of the Department of Neurodegenerative Diseases and Geriatric Psychiatry at the University Hospital Bonn and conducts research there and at the DNZE on neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease.
Division of labor prevents overload
The research suggests that microglial cells may spontaneously join together in order to better cope with threats. For this purpose, they form tube-like projections that dock onto neighboring microglial cells. These connections are then used to distribute the aSyn aggregates among the partners in the network. Without this division of labor, individual immune cells would have to shoulder a major part of the degradation work and would be overwhelmed.
Joining forces prevents that from happening. However, the connecting tubes also serve another purpose: Microglial cells can use them to give their neighbors a boost when they are in too much distress or indeed in mortal danger. “They then send mitochondria to neighboring cells that are busy breaking down the aggregates,” explains Heneka’s colleague Dr. Hannah Scheiblich. “Mitochondria function like little power plants; so they provide extra energy to the stressed cells.”
In certain mutations, which are found more frequently in Parkinson’s disease patients, both aSyn and mitochondrial transport are impaired. A similar situation applies to another disease in which the degradation of aSyn is impaired: Lewy body dementia. Researchers have isolated certain immune cells, the macrophages, from blood samples of affected individuals. These can be converted into microglia-like cells with the help of specific regulatory molecules. “These were still able to form networks in the lab. However, the transport of aSyn through the connecting tubes was severely impaired,” says Heneka, who is also a member of the Cluster of Excellence ImmunoSensation2 and the transdisciplinary research area “Life & Health”.
Findings may open up new therapeutic perspectives
The fact that microglial cells can join together was previously unknown. “We have opened the door to a field that will certainly engage researchers for many years to come,” Heneka emphasizes. In the medium term, this may also open up new therapeutic perspectives for neurological disorders such as Parkinson’s disease or dementia.
(Image caption: Microglial cells - (blue: the cell nuclei) can join together using tubular projections (red) to degrade dangerous proteins in a division of labor. Credit: © AG Heneka/University of Bonn)
Brain Tissue Inflammation Drives Alzheimer’s Disease
Neuroinflammation is the key driver of the spread of pathologically misfolded proteins in the brain and causes cognitive impairment in patients with Alzheimer’s disease, researchers from the University of Pittsburgh School of Medicine reveal in a paper published in Nature Medicine.
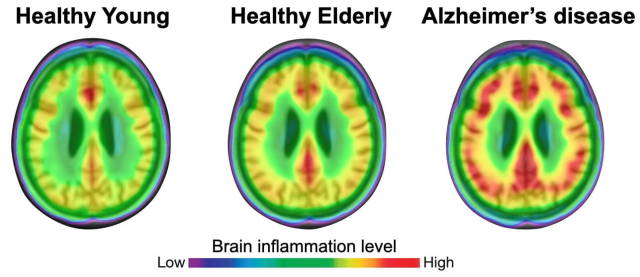
(Image caption: The degree of neuroinflammation (red) is more pronounced in brains of patients with Alzheimer’s disease than in healthy individuals. Credit: Adapted from Pascoal et al., Nature Medicine)
For the first time ever, the researchers showed in living patients that neuroinflammation—or activation of the brain’s resident immune cells, called microglial cells—is not merely a consequence of disease progression; rather, it is a key upstream mechanism that is indispensable for disease development.
“As a young resident neurologist in my home country of Brazil, I noticed that many patients with Alzheimer’s disease are left neglected and without access to appropriate care,” said lead author Tharick Pascoal, M.D., Ph.D., assistant professor of psychiatry and neurology at Pitt. “Our research suggests that combination therapy aimed to reduce amyloid plaque formation and limit neuroinflammation might be more effective than addressing each pathology individually.”
Alzheimer’s disease is characterized by the accumulation of amyloid plaques—protein aggregates lodged between nerve cells of the brain—and clumps of disordered protein fibers, called tau tangles, forming inside the nerve cells. Although studies in cultured cells and lab animals amassed ample evidence that microglial activation drives the spread of tau fibers in Alzheimer’s disease, this process has never been proven in humans.
The study findings suggest that targeting neuroinflammation might be beneficial for people with early-stage Alzheimer’s disease and that it might help reverse or at least slow down the accumulation of pathologic tau protein in the brain and stave off dementia.
To determine the mechanism by which disordered tangles of tau protein fibers and amyloid plaques spread across the brain and lead to dementia, the researchers used live imaging to look deep into the brains of people with various stages of Alzheimer’s disease and healthy aging individuals.
The researchers found that neuroinflammation was more prevalent in older people and that it was even more pronounced in patients with mild cognitive impairments and those with Alzheimer’s disease-associated dementia. Bioinformatics analysis confirmed that tau propagation depended on microglial activation—it is a key element that links the effects of amyloid plaque aggregation to tau spread and, ultimately, cognitive impairment and dementia.
“Many elderly people have amyloid plaques in their brains but never progress to developing Alzheimer’s disease,” said Pascoal. “We know that amyloid accumulation on its own is not enough to cause dementia—our results suggest that it is the interaction between neuroinflammation and amyloid pathology that unleashes tau propagation and eventually leads to wide-spread brain damage and cognitive impairment.”