#parkinsons disease
The active ingredient in an over-the-counter skin cream might do more than prevent wrinkles. Scientists have discovered that the drug, called kinetin, also slows or stops the effects of Parkinson’s disease on brain cells.
Scientists identified the link through biochemical and cellular studies, but the research team is now testing the drug in animal models of Parkinson’s. The research is published in the August 15, 2013 issue of the journal Cell.
“Kinetin is a great molecule to pursue because it’s already sold in drugstores as a topical anti-wrinkle cream,” says HHMI investigator Kevan Shokat of the University of California, San Francisco. “So it’s a drug we know has been in people and is safe.”
Parkinson’s disease is a degenerative disease that causes the death of neurons in the brain. Initially, the disease affects one’s movement and causes tremors, difficulty walking, and slurred speech. Later stages of the disease can cause dementia and broader health problems. In 2004, researchers studying an Italian family with a high prevalence of early-onset Parkinson’s disease discovered mutations in a protein called PINK1 associated with the inherited form of the disease.
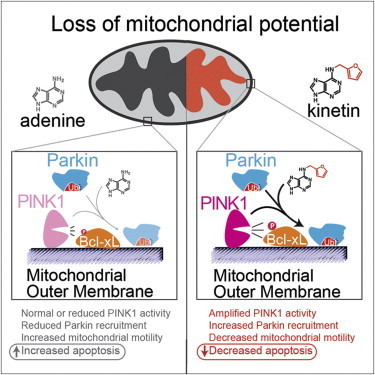
Since then, studies have shown that PINK1 normally wedges into the membrane of damaged mitochondria inside cells that causes another protein, Parkin, to be recruited to the mitochondria, which are organelles responsible for energy generation. Neurons require high levels of energy production, therefore when mitochondrial damage occurs, it can lead to neuronal death. However, when Parkin is present on damaged mitochondria, studding the mitochondrial surface, the cell is able to survive the damage. In people who inherit mutations in PINK1, however, Parkin is never recruited to the organelles, leading to more frequent neuronal death than usual.
Shokat and his colleagues wanted to develop a way to turn on or crank up PINK1 activity, therefore preventing an excess of cell death, in those with inherited Parkinson’s disease. But turning on activity of a mutant enzyme is typically more difficult than blocking activity of an overactive version.
“When we started this project, we really thought that there would be no conceivable way to make something that directly turns on the enzyme,” says Shokat. “For any enzyme we know that causes a disease, we have ways to make inhibitors but no real ways to turn up activity.”
His team expected it would have to find a less direct way to mimic the activity of PINK1 and recruit Parkin. In the hopes of more fully understanding how PINK1 works, they began investigating how PINK1 binds to ATP, the energy molecule that normally turns it on. In one test, instead of adding ATP to the enzymes, they added different ATP analogues, versions of ATP with altered chemical groups that slightly change its shape. Scientists typically must engineer new versions of proteins to be able to accept these analogs, since they don’t fit into the typical ATP binding site. But to Shokat’s surprise, one of the analogs—kinetin triphosphate, or KTP—turned on the activity of not only normal PINK1, but also the mutated version, which doesn’t bind ATP.
“This drug does something that chemically we just never thought was possible,” says Shokat. “But it goes to show that if you find the right key for the right lock, you’ll be able to open the door.”
To test whether the binding of KTP to PINK1 led to the same consequences as the usual ATP binding, Shokat’s group measured the activity of PINK1 directly, as well as the downstream consequences of this activity, including the amount of Parkin recruited to the mitochondrial surface, and the levels of cell death. Adding the precursor of KTP, kinetin, to cells—both those with PINK1 mutations and those with normal physiology—amplified the activity of PINK1, increased the level of Parkin on damaged mitochondria, and decreased levels of neuron death, they found.
“What we have here is a case where the molecular target has been shown to be important to Parkinson’s in human genetic studies,” says Shokat. “And now we have a drug that specifically acts on this target and reverses the cellular causes of the disease.”
The similar results in cells with and without PINK1 mutations suggest that kinetin, which is a precursor to KTP, could be used to treat not only Parkinson’s patients with a known PINK1 mutation, but to slow progression of the disease in those without a family history by decreasing cell death.
Shokat is now performing experiments on the effects of kinetin in mice with various forms of Parkinson’s disease. However, the usefulness of animal models in Parkinson’s research has been debated, and therefore the positive results from the cellular data, he says, is as good an indicator as results in animals that this drug has potential to treat Parkinson’s in humans. Initial human studies will likely focus on the small population of patients with PINK1 mutations, and if successful in that group the drug could later be tested in a wider array of Parkinson’s patients.
Research Shows Promising Results for Parkinson’s Disease Treatment
Researchers from Carnegie Mellon University have found a way to make deep brain stimulation (DBS) more precise, resulting in therapeutic effects that outlast what is currently available. The work, led by Aryn Gittis and colleagues in CMU’s Gittis Lab and published in Science, will significantly advance the study of Parkinson’s disease.
DBS allows researchers and doctors to use thin electrodes implanted in the brain to send electrical signals to the part of the brain that controls movement. It is a proven way to help control unwanted movement in the body, but patients must receive continuous electrical stimulation to get relief from their symptoms. If the stimulator is turned off, the symptoms return immediately.
Gittis, an associate professor of biological sciences in the Mellon College of Science and faculty in the Neuroscience Institute, said that the new research could change that.
“By finding a way to intervene that has long-lasting effects, our hope is to greatly reduce stimulation time, therefore minimizing side effects and prolonging battery life of implants.”
Gittis set the foundation for this therapeutic approach in 2017, when her lab identified specific classes of neurons within the brain’s motor circuitry that could be targeted to provide long-lasting relief of motor symptoms in Parkinson’s models. In that work, the lab used optogenetics, a technique that uses light to control genetically modified neurons. Optogenetics, however, cannot currently be used on humans.
Since then, she has been trying to find a strategy that is more readily translated to patients suffering from Parkinson’s disease. Her team found success in mice with a new DBS protocol that uses short bursts of electrical stimulation.
“This is a big advance over other existing treatments,” Gittis said. “In other DBS protocols, as soon as you turn the stimulation off, the symptoms come back. This seems to provide longer lasting benefits — at least four times longer than conventional DBS.”
In the new protocol, the researchers target specific neuronal subpopulations in the globus pallidus, an area of the brain in the basal ganglia, with short bursts of electrical stimulation. Gittis said that researchers have been trying for years to find ways to deliver stimulation in such a cell-type specific manner.
“That concept is not new. We used a ‘bottom up’ approach to drive cell type specificity. We studied the biology of these cells and identified the inputs that drive them. We found a sweet spot that allowed us to utilize the underlying biology,” she said.
Teresa Spix, the first author of the paper, said that while there are many strong theories, scientists do not yet fully understand why DBS works.
“We’re sort of playing with the black box. We don’t yet understand every single piece of what’s going on in there, but our short burst approach seems to provide greater symptom relief. The change in pattern lets us differentially affect the cell types,” she said.
Spix, who defended her Ph.D. in July, is excited about the direct connection this research has to clinical studies.
“A lot of times those of us that work in basic science research labs don’t necessarily have a lot of contact with actual patients. This research started with very basic circuitry questions but led to something that could help patients in the near future,” Spix said.
Next, neurosurgeons at Pittsburgh’s Allegheny Health Network (AHN) will use Gittis’ research in a safety and tolerability study in humans. Nestor Tomycz, a neurological surgeon at AHN, said that researchers will soon begin a randomized, double blind crossover study of patients with idiopathic Parkinson’s disease. The patients will be followed for 12 months to assess improvements in their Parkinson’s disease motor symptoms and frequency of adverse events.
“Aryn Gittis continues to do spectacular research which is elucidating our understanding of basal ganglia pathology in movement disorders. We are excited that her research on burst stimulation shows a potential to improve upon DBS which is already a well-established and effective therapy for Parkinson’s disease,” Tomycz said.
Donald Whiting, the chief medical officer at AHN and one of the nation’s foremost experts in the use of DBS, said the new protocol could open doors for experimental treatments.
“Aryn is helping us highlight in the animal model things that are going to change the future of what we do for our patients. She’s actually helping evolve the care treatment of Parkinson’s patients for decades to come with her research,” Whiting said.
Tomycz agreed. “This work is really going to help design the future technology that we’re using in the brain and will help us to get better outcomes for these patients.”

Immune cells in the brain share the work
To break down toxic proteins more quickly, immune cells in the brain can join together to form networks when needed. This is shown by a joint study of the University of Bonn, the German Center for Neurodegenerative Diseases (DZNE) and the Institut François Jacob in France. However, in certain mutations that can cause Parkinson’s disease, this cooperation is impaired. The findings are published in the renowned journal Cell.
The protein alpha-synuclein (abbreviated aSyn) performs important tasks in the nerve cells of the brain. But under certain circumstances, aSyn molecules can clump together and form insoluble aggregates. These damage the neurons; they are for instance typically found in the brains of people suffering from Parkinson’s disease or Lewy body dementia.
The immune cells of the brain, the microglial cells, therefore try to break down and dispose of the aSyn aggregates. This process is not only time-consuming; it can also cause the microglial cells themselves to perish. “We have now identified a mechanism that addresses both problems,” explains Prof. Dr. Michael Heneka. The researcher is director of the Department of Neurodegenerative Diseases and Geriatric Psychiatry at the University Hospital Bonn and conducts research there and at the DNZE on neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease.
Division of labor prevents overload
The research suggests that microglial cells may spontaneously join together in order to better cope with threats. For this purpose, they form tube-like projections that dock onto neighboring microglial cells. These connections are then used to distribute the aSyn aggregates among the partners in the network. Without this division of labor, individual immune cells would have to shoulder a major part of the degradation work and would be overwhelmed.
Joining forces prevents that from happening. However, the connecting tubes also serve another purpose: Microglial cells can use them to give their neighbors a boost when they are in too much distress or indeed in mortal danger. “They then send mitochondria to neighboring cells that are busy breaking down the aggregates,” explains Heneka’s colleague Dr. Hannah Scheiblich. “Mitochondria function like little power plants; so they provide extra energy to the stressed cells.”
In certain mutations, which are found more frequently in Parkinson’s disease patients, both aSyn and mitochondrial transport are impaired. A similar situation applies to another disease in which the degradation of aSyn is impaired: Lewy body dementia. Researchers have isolated certain immune cells, the macrophages, from blood samples of affected individuals. These can be converted into microglia-like cells with the help of specific regulatory molecules. “These were still able to form networks in the lab. However, the transport of aSyn through the connecting tubes was severely impaired,” says Heneka, who is also a member of the Cluster of Excellence ImmunoSensation2 and the transdisciplinary research area “Life & Health”.
Findings may open up new therapeutic perspectives
The fact that microglial cells can join together was previously unknown. “We have opened the door to a field that will certainly engage researchers for many years to come,” Heneka emphasizes. In the medium term, this may also open up new therapeutic perspectives for neurological disorders such as Parkinson’s disease or dementia.
(Image caption: Microglial cells - (blue: the cell nuclei) can join together using tubular projections (red) to degrade dangerous proteins in a division of labor. Credit: © AG Heneka/University of Bonn)

How a Parkinson’s disease-linked protein attacks a cell’s powerhouses
Inside cells, organelles called mitochondria carry out a medley of vital tasks. These structures generate energy and help to keep the cells’ interior environment in a state of healthy equilibrium, among other functions.
Now, scientists show how a protein associated with Parkinson’s disease can damage these cellular powerhouses.
The findings come from experiments in which fruit fly larvae were genetically engineered to produce unusually high amounts of the protein, called alpha-synuclein.
“When fruit fly larvae expressed alpha-synuclein at elevated levels similar to what is seen in Parkinson’s disease, many of the mitochondria we observed became unhealthy, and many became fragmented. Through detailed experiments, we also showed that different parts of the alpha-synuclein protein seem to be responsible for these two problems, and that fragmented mitochondria can actually be healthy. This is a key finding, because before, people thought fragmented mitochondria were unhealthy mitochondria,” says Shermali Gunawardena, PhD, associate professor of biological sciences in the University at Buffalo College of Arts and Sciences.
The results could be of interest in the context of drug development, as abnormal aggregates of alpha-synuclein in brain cells are a hallmark of Parkinson’s disease, and mitochondrial damage has also been observed in patients.
“This research showcases the advantage of using fruit fly larvae as a model organism to study how neurons become damaged during devastating diseases such as Parkinson’s disease,” says TJ Krzystek, UB PhD candidate in biological sciences. “Through this approach, we pieced together a new understanding for how the Parkinson’s disease-related protein alpha-synuclein disrupts the health and movement of mitochondria — the epicenter for energy production in cells. We believe this work emphasizes a promising path that can be explored for potential therapeutics aimed at improving mitochondrial health in Parkinson’s disease patients.”
The study was published in the journal Cell Death and Disease.
The co-first authors are Krzystek and Rupkatha Banerjee, PhD, a postdoctoral research associate at Scripps Research who completed her doctorate in biological sciences at UB. Gunawardena is the senior author.
The research was a collaborative effort, with many members of the Gunawardena lab making significant contributions. In addition to Banerjee, Gunawardena and Krzystek, the paper’s authors include undergraduates Layne Thurston, JianQiao Huang and Saad Navid Rahman, and PhD student Kelsey Swinter, all in the UB Department of Biological Sciences, and Tomas L. Falzone at the Universidad de Buenos Aires and Instituto de Investigación en Biomedicina de Buenos Aires.
A detailed look at alpha-synuclein and mitochondria
Through tests in fruit fly larvae, the scientists were able to tease out intricate details regarding interactions between alpha-synuclein and mitochondria.
For example, the study not only concludes that different sections of the alpha-synuclein protein are likely responsible for causing mitochondrial fragmentation and damaging mitochondrial health; the research also identifies these sections and describes how other proteins may interact with them to drive these changes. More specifically, the proteins PINK1 and Parkin — both linked to Parkinson’s disease — may interact with one end of alpha-synuclein to influence mitochondrial health, while a protein called DRP1 may interact with the other end to break mitochondria, scientists say.
“Mitochondrial impairments have long been linked to the pathogenesis of Parkinson’s disease,” Banerjee says. “However, the role of alpha-synuclein in mitochondrial quality control so far has not been comprehensively investigated. Our study unravels the intricate molecular mechanisms by which the different regions of alpha-synuclein exert distinct effects on mitochondrial health, bringing into light a potential pathway that could be targeted for exploring new therapeutic interventions in Parkinson’s disease.”
“We were able to tease out specific mechanistic functions for alpha synuclein by using imaging tools and a color-tagged marking system to observe the process of what happens to mitochondria when alpha-synuclein is elevated,” Gunawardena adds. “This system allowed us to observe the health, size and the movement behaviors of mitochondria at the same time in living neurons in a whole organism.”
(Image caption: Unhealthy mitochondria are marked in a gradient from white to red, with white being the least healthy, in contrast to healthy mitochondria that appear in blue. This still image is from a microscope video showing mitochondria moving in a fruit fly larval neuron expressing elevated levels of the protein alpha-synuclein. Credit: TJ Krzystek and Shermali Gunawardena)
Brain Cell Transplants Are Being Tested Once Again For Parkinson’s
by Jon Hamilton / NPR Health
Researchers are working to revive a radical treatment for Parkinson’s disease.
The treatment involves transplanting healthy brain cells to replace cells killed off by the disease. It’s an approach that was tried decades ago and then set aside after disappointing results.
Now, groups in Europe, the U.S. and Asia are preparing to try again, using cells they believe are safer and more effective.
“There have been massive advances,” says Claire Henchcliffe, a neurologist at Weill Cornell Medicine in New York. “I’m optimistic.”
“We are very optimistic about ability of [the new] cells to improve patients’ symptoms,” says Viviane Tabar, a neurosurgeon and stem cell biologist at Memorial Sloan Kettering Cancer Center in New York.
Henchcliffe and Tabar joined several other prominent scientists to describe plans to revive brain cell transplants during a session Tuesday at the International Society for Stem Cell Research meeting in Boston.
Their upbeat message marks a dramatic turnaround for the approach.
During the 1980s and 1990s, researchers used cells taken directly from the brains of aborted fetuses to treat hundreds of Parkinson’s patients. The goal was to halt the disease.
Parkinson’s destroys brain cells that make a substance called dopamine. Without enough dopamine, nerve cells can’t communicate with muscles, and people can develop tremors, have difficulty walking and other symptoms.
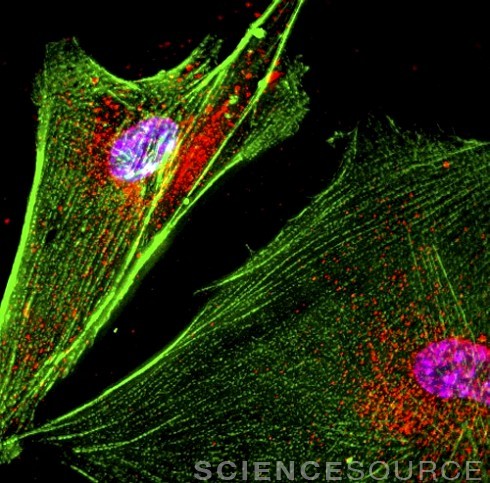
Image above © Roger J. Bick & Brian J. Poindexter / Science Source
Post link
I haven’t posted in a while, there are good reasons for that. I have been dealing with what I thought was a condition known as Essential Tremor.
I had a pain specialist that was trying to find a way not to treat me because he didn’t like my chances for “getting better.” He has since closed his practice and I had to see my original doctor that I saw in 2014, the one I began this long and very odd journey with. Unfortunately that doctor has decided that since he now doesn’t know what is wrong with me he can’t treat me either, I wish he would have decided that before I spent the time and energy getting a psychological test that told everyone I’m not suicidal nor an addiction waiting to happen. My word wasn’t good enough. Did I mention insurance didn’t cover this test?
I went to see my neurologist around October and she decided that my diagnosis may be wrong and I look more like a Parkinson’s patient to her, she said I needed to be seen by a movement specialist and she would send my records to the UofM and get me an appointment, it would probably take a few weeks to hear back. When I called in December to see if there were any updates, I was told the records had not been sent yet, all of that waiting was for nothing and I had to wait until the holidays were over to have them sent. They eventually made it out of the office on January 5th. When I called the UofM I was told I got my appointment with them, but it isn’t until October 24th (it is still February) so I have to wait.
While the waiting is happening I am continuing to get worse, now to go with the tremors and foot drop, I have speech problems. Some days my words don’t come out (I can’t talk at all), I have trouble finding words, and the newest fun is the stutter that makes everything more difficult.
I decided to try and get an appointment sooner so I called the University of Toledo and their first available appointment is in April, still a wait but much better than October.
I am going to keep moving and hope that when I get to these clinics they have answers, I need to know what has been taking my ability to hold still and talk.
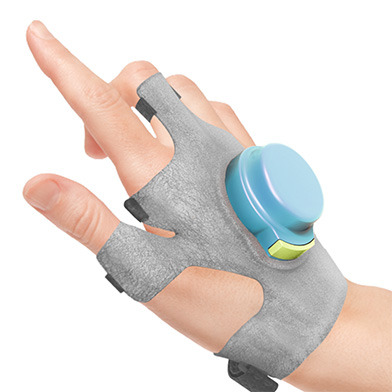
How the GyroGlove Steadies Hands of Parkinson’s Patients
When he was a 24-year-old medical student living in London, Faii Ong was assigned to care for a 103-year-old patient who suffered from Parkinson’s, the progressive neurological condition that affects a person’s ease of movement. After watching her struggle to eat a bowl of soup, Ong asked another nurse what more could be done to help the woman. “There’s nothing,” he was grimly told.
GyroGlove’sdesign is simple. It uses a miniature, dynamically adjustable gyroscope, which sits on the back of the hand, within a plastic casing attached to the glove’s material. When the device is switched on, the battery-powered gyroscope whirs to life. Its orientation is adjusted by a precession[sic] hinge and turntable, both controlled by a small circuit board, thereby pushing back against the wearer’s movements as the gyroscope tries to right itself.
Post link