#neuroinflammation
Brain Tissue Inflammation Drives Alzheimer’s Disease
Neuroinflammation is the key driver of the spread of pathologically misfolded proteins in the brain and causes cognitive impairment in patients with Alzheimer’s disease, researchers from the University of Pittsburgh School of Medicine reveal in a paper published in Nature Medicine.
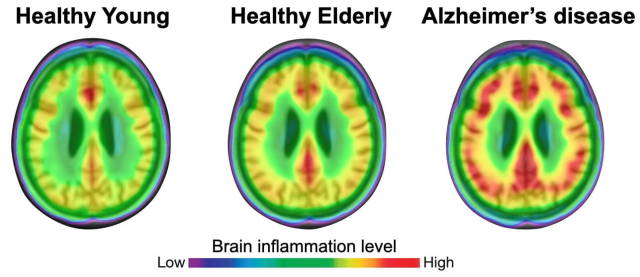
(Image caption: The degree of neuroinflammation (red) is more pronounced in brains of patients with Alzheimer’s disease than in healthy individuals. Credit: Adapted from Pascoal et al., Nature Medicine)
For the first time ever, the researchers showed in living patients that neuroinflammation—or activation of the brain’s resident immune cells, called microglial cells—is not merely a consequence of disease progression; rather, it is a key upstream mechanism that is indispensable for disease development.
“As a young resident neurologist in my home country of Brazil, I noticed that many patients with Alzheimer’s disease are left neglected and without access to appropriate care,” said lead author Tharick Pascoal, M.D., Ph.D., assistant professor of psychiatry and neurology at Pitt. “Our research suggests that combination therapy aimed to reduce amyloid plaque formation and limit neuroinflammation might be more effective than addressing each pathology individually.”
Alzheimer’s disease is characterized by the accumulation of amyloid plaques—protein aggregates lodged between nerve cells of the brain—and clumps of disordered protein fibers, called tau tangles, forming inside the nerve cells. Although studies in cultured cells and lab animals amassed ample evidence that microglial activation drives the spread of tau fibers in Alzheimer’s disease, this process has never been proven in humans.
The study findings suggest that targeting neuroinflammation might be beneficial for people with early-stage Alzheimer’s disease and that it might help reverse or at least slow down the accumulation of pathologic tau protein in the brain and stave off dementia.
To determine the mechanism by which disordered tangles of tau protein fibers and amyloid plaques spread across the brain and lead to dementia, the researchers used live imaging to look deep into the brains of people with various stages of Alzheimer’s disease and healthy aging individuals.
The researchers found that neuroinflammation was more prevalent in older people and that it was even more pronounced in patients with mild cognitive impairments and those with Alzheimer’s disease-associated dementia. Bioinformatics analysis confirmed that tau propagation depended on microglial activation—it is a key element that links the effects of amyloid plaque aggregation to tau spread and, ultimately, cognitive impairment and dementia.
“Many elderly people have amyloid plaques in their brains but never progress to developing Alzheimer’s disease,” said Pascoal. “We know that amyloid accumulation on its own is not enough to cause dementia—our results suggest that it is the interaction between neuroinflammation and amyloid pathology that unleashes tau propagation and eventually leads to wide-spread brain damage and cognitive impairment.”
E-cigarettes Alter Inflammatory State of Brain, Heart, Lungs and Colon
Daily use of pod-based e-cigarettes alters the inflammatory state of multiple organ systems including the brain, heart, lungs and colon, according to a new study done in mice from researchers at University of California San Diego School of Medicine, published April 12, 2022 in the journal eLife. Effects also vary depending on the e-cigarette flavor and can influence how organs respond to infections, such as SARS-CoV-2.
Authors saw the most striking effects in the brain, where several inflammatory markers were elevated. Additional changes in neuroinflammatory gene expression were noted in the nucleus accumbens, a brain region critical for motivation and reward-processing. The findings raise major concerns, they said, as neuroinflammation in this region has been linked to anxiety, depression and addictive behaviors, which could further exacerbate substance use and addiction.
“Many JUUL users are adolescents or young adults whose brains are still developing, so it’s pretty terrifying to learn what may be happening in their brains considering how this could affect their mental health and behavior down the line,” said senior study author Laura Crotty Alexander, MD, associate professor of medicine at UC San Diego School of Medicine.
The researchers also found that the inflammatory response of each organ varied depending on which JUUL flavor was used. For example, the hearts of mice that inhaled mint aerosols were much more sensitive to the effects of bacterial pneumonia compared to those that inhaled mango aerosols.
“This was a real surprise to us,” said Crotty Alexander. “This shows us that the flavor chemicals themselves are also causing pathological changes. If someone who frequently uses menthol-flavored JUUL e-cigarettes was infected with COVID-19, it’s possible their body would respond differently to the infection.”
While mint and mango JUUL flavors have been discontinued since this study began, many of their chemical ingredients can still be found in current JUUL products, such as their menthol flavor, or other brands of flavored e-cigarettes.
— Nicole Mlynaryk
Post link

