#okayama university
Trapping gases better with boron nitride ‘nanopores’
What is common between a technology for storing energy in a solar cell and that for water purification? They both rely on the use of porous materials, or more specifically, 'nanoporous’ materials that can trap gas molecules within narrow spaces on their surface, called pores, which are only nanometers (one-billionth of a meter) in size. In the parlance of chemistry, the phenomenon is known as adsorption and has played an important role in the synthesis of porous materials of different compositions, pore sizes, and even pore geometries.
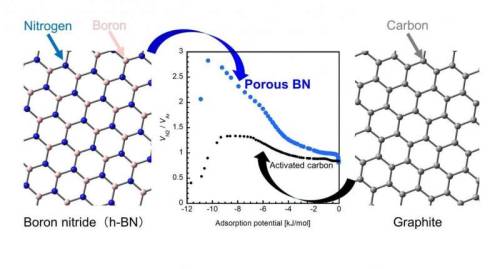
Traditionally, activated carbon (AC, or a porous form of carbon) has been a popular adsorbent for practical applications owing to its higher capacity of adsorption than that of other porous materials. Lately, however, porous boron nitride (p-BN) has emerged as a promising alternative because of its impressive performance, as highlighted by a recent study claiming that p-BN can adsorb a relatively large amount of carbon dioxide at room temperature.
Now, a group of scientists from Okayama University and Nagasaki University, Japan, has put this claim to the test in their latest study, where they examined the adsorbing characteristics of p-BN in detail. “A BN unit and two carbon atoms (i.e., CC) both have the same number of electrons and similar structures, but their interaction with gas molecules are different due to the atomically heterogeneous nature of BN. Despite this, there has been very little research on BN materials. In our study, we wanted to see if BN has specific adsorption properties that cannot be observed in carbon materials,” explains Dr. Takahiro Ohkubo from Okayama University, who led this study published in the journal RSC Advances.
Post link