#porosity
Simple logic for nanofluidic computing simulated
Invigorating the idea of computers based on fluids instead of silicon, researchers at the National Institute of Standards and Technology (NIST) have shown how computational logic operations could be performed in a liquid medium by simulating the trapping of ions (charged atoms) in graphene (a sheet of carbon atoms) floating in saline solution. The scheme might also be used in applications such as water filtration, energy storage or sensor technology.
The idea of using a liquid medium for computing has been around for decades, and various approaches have been proposed. Among its potential advantages, this approach would require very little material and its soft components could conform to custom shapes in, for example, the human body.
NIST’s ion-based transistor and logic operations are simpler in concept than earlier proposals. The new simulations show that a special film immersed in liquid can act like a solid silicon-based semiconductor. For example, the material can act like a transistor, the switch that carries out digital logic operations in a computer. The film can be switched on and off by tuning voltage levels like those induced by salt concentrations in biological systems.
Post link
Trapping gases better with boron nitride ‘nanopores’
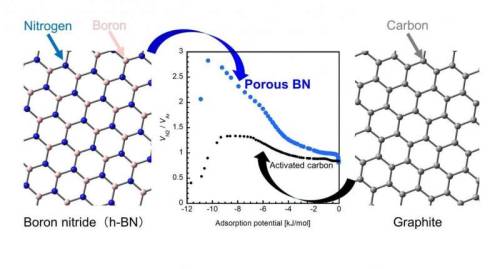
What is common between a technology for storing energy in a solar cell and that for water purification? They both rely on the use of porous materials, or more specifically, 'nanoporous’ materials that can trap gas molecules within narrow spaces on their surface, called pores, which are only nanometers (one-billionth of a meter) in size. In the parlance of chemistry, the phenomenon is known as adsorption and has played an important role in the synthesis of porous materials of different compositions, pore sizes, and even pore geometries.
Traditionally, activated carbon (AC, or a porous form of carbon) has been a popular adsorbent for practical applications owing to its higher capacity of adsorption than that of other porous materials. Lately, however, porous boron nitride (p-BN) has emerged as a promising alternative because of its impressive performance, as highlighted by a recent study claiming that p-BN can adsorb a relatively large amount of carbon dioxide at room temperature.
Now, a group of scientists from Okayama University and Nagasaki University, Japan, has put this claim to the test in their latest study, where they examined the adsorbing characteristics of p-BN in detail. “A BN unit and two carbon atoms (i.e., CC) both have the same number of electrons and similar structures, but their interaction with gas molecules are different due to the atomically heterogeneous nature of BN. Despite this, there has been very little research on BN materials. In our study, we wanted to see if BN has specific adsorption properties that cannot be observed in carbon materials,” explains Dr. Takahiro Ohkubo from Okayama University, who led this study published in the journal RSC Advances.
Post link
Advanced NMR captures new details in nanoparticle structures
Advanced nuclear magnetic resonance (NMR) techniques at the U.S. Department of Energy’s Ames Laboratory have revealed surprising details about the structure of a key group of materials in nanotechology, mesoporous silica nanoparticles (MSNs), and the placement of their active chemical sites.
MSNs are honeycombed with tiny (about 2-15 nm wide) three-dimensionally ordered tunnels or pores, and serve as supports for organic functional groups tailored to a wide range of needs. With possible applications in catalysis, chemical separations, biosensing, and drug delivery, MSNs are the focus of intense scientific research.
“Since the development of MSNs, people have been trying to control the way they function,” said Takeshi Kobayashi, an NMR scientist with the Division of Chemical and Biological Sciences at Ames Laboratory. “Research has explored doing this through modifying particle size and shape, pore size, and by deploying various organic functional groups on their surfaces to accomplish the desired chemical tasks. However, understanding of the results of these synthetic efforts can be very challenging.”
Post link
New nanocrystals put a tiny twist on useful materials
A new kind of tiny particle is a big deal in UO chemist Carl Brozek’s lab.
He and his team have made a versatile kind of porous material called a metal-organic framework, or MOF, into nanocrystals—a form that’s easier to use beyond the lab. Nanoparticles such as these have a wide range of potential applications, from surface coatings that can store electric charge, to filters that remove contaminants from air or water.
The nanocrystals are the smallest and most stable MOFs made yet, said Brozek. And they have an array of interesting properties—notably, they can conduct electricity, and they behave differently depending on the exact size of the particle.
“It really feels like we’ve cracked into something new,” Brozek said. He and his team, led by graduate student Checkers Marshall, reported their advance November 24 in a pre-print posted to the research site ChemRxiv.
Post link
Corralling xenon gas out of waste streams
From space propulsion to lighting to surgical anesthesia, the applications and needs for xenon gas are growing. And the good news is that researchers are advancing the science to more easily remove xenon from waste streams and collect the low amounts of it found in the atmosphere.
Researchers at the Department of Energy’s Pacific Northwest National Laboratory are at the forefront of research developing porous nanoscale materials to capture xenon. They report in the journal Chem this month, that inexpensive materials called metal-organic frameworks have been very successful in separating the gas in a way that may make it far less expensive than existing means of producing it.
Currently, industry uses a common but expensive process called cryogenic distillation to separate xenon from other gases or the atmosphere. In that costly process, a lot of energy is used to chill entire gas streams down to far below freezing in order to concentrate the xenon.
Post link
Novel photoresist enables 3D printing of smallest porous structures
Researchers of the cluster of excellence 3D matter made to order expand possibilities of two-photon microprinting
Researchers of Karlsruhe Institute of Technology (KIT) and Heidelberg University have developed a photoresist for two-photon microprinting. It has now been used for the first time to produce three-dimensional polymer microstructures with cavities in the nano range. In Advanced Materials, the scientists involved in the joint Cluster of Excellence 3D Matter Made to Order report how porosity can be controlled during printing and how this affects light scattering properties of the microstructures.
Photoresists are printing inks used to print smallest microstructures in three dimensions by so-called two-photon lithography. During printing, a laser beam is moved in all spatial directions through the initially liquid photoresist. The photoresist hardens in the focal point of the laser beam only. Little by little, complex microstructures can be built in this way. In a second step, a solvent is used to remove those areas that were not exposed to radiation. Complex polymer architectures in the micrometer and nanometer ranges remain.
Two-photon polymerization – or two-photon microprinting based on this process – has been studied extensively for some years now, in particular as regards the production of microoptics, so-called metamaterials, and microscaffolds for experiments with single biological cells. To expand the spectrum of applications, new printable materials are required. This is the point of departure of the scientists involved in the Cluster of Excellence 3D Matter Made to Order (3DMM2O) of KIT and Heidelberg University. “With the help of conventional photoresists, it was possible to print transparent, glassy polymers only,” says Frederik Mayer, physicist of KIT and main author of the study. “Our new photoresist for the first time enables printing of 3D microstructures from porous nanofoam. This polymer foam has cavities of 30 to 100 nm in size, which are filled with air.”