#perfumery
What smells do you associate with “cleanness”? Is it the clean, dewy freshness after a thunderstorm (due to geosmin - Day 14)? Or is it that warm, enveloping smoothness of clean linen?
If you’re thinking about the latter, Galaxolide (C18H26O) is probably responsible for that. It belongs to a class of compounds called “musks”, which are extremely commonly used in fragrances, from functional (detergents and soaps) to fine (perfumes).
Musks used to be obtained from musk pods - a gland of musk deer - since ancient times, when they were discovered to not only have an aroma of their own, but also acted as a fixative for fragrances, which is to say that it prolonged the lasting power of fragrances that it was added to. Natural musk can also be obtained from civet cats and muskrats, amongst other animals.

However, in recent decades, the industry has turned to synthetic musks due to sustainability and ethical concerns regarding the harvesting of musk pods.
The first synthetic musks were discovered serendipitously in the late 19th century; a chemist, Albert Bauer, was trying to find a more explosive analogue to trinitrotoluene (TNT - Day 58) when he noticed that the products of his experiments had a pleasant musky odour. These “nitro musks”, as they were called, found widespread use due to their relatively low costs and similarity to natural musk. Chanel famously used nitro musks in many of their perfumes, including No. 5.
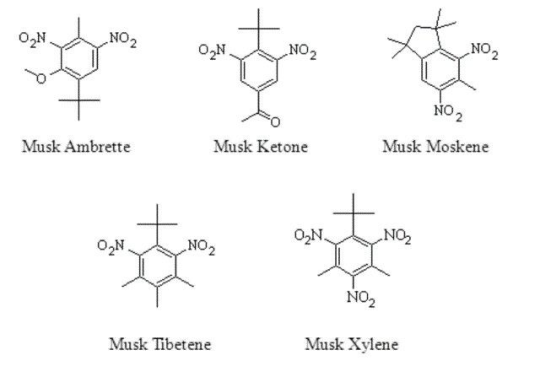
However, nitro musks were eventually suspected of neurotoxicity, and were phased out; only one nitro musk, musk ketone, is currently approved to be used in the fragrance industry.
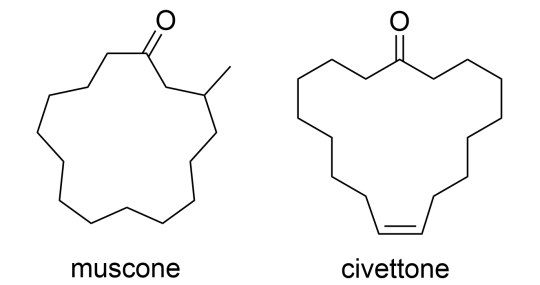
Other types of musks - macrocyclic musks, such as muscone and civettone, were isolated from natural musk from musk deer and civet cats. However, industrial synthesis proved too complicated and commercially unfeasible for the early 20th century.
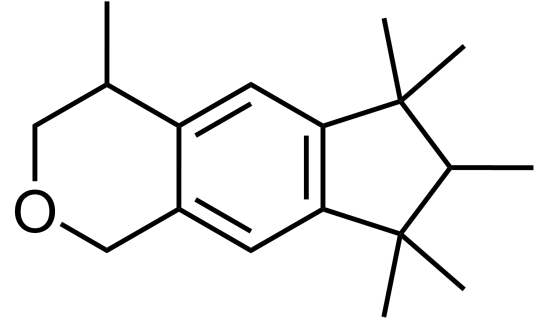
The hunt for a new type of synthetic musk - one that was safe and cheap - was on again. In 1965, Galaxolide was first synthesised by International Flavors and Fragrances (IFF) in an attempt to make existing musks more chemically stable and hydrophobic, and was quickly utilised in many functional and fine fragrances due to its crisp, clean connotations; it was completely free of any animalic nuances, unlike molecules like muscone.
Why does the molecule have to be hydrophobic? Consider its purpose - when used in detergents, it would be a desirable quality to be able to adsorb onto fabric fibres and not be washed off by water. Its relatively large size meant that strong intermolecular forces of attractions would lead to low volatility too, thus allowing it to impart a long-lasting pleasant smell.

While Galaxolide was not the first polycyclic musk to be discovered, it is by far the most well-known one and is produced on a scale of 1,000 tons a year as of 2015.
However, with its ubiquity, its persistence in nature and its tendency to bioaccumulate has been brought under scrutiny. While no toxic effects to humans have been reported, it has still been a cause for concern as its effects on wildlife are not well understood.
And the search for new affordable, safe, and biodegradable musks continues…
My dear readers - what chemicals have you tried sniffing? Which ones had a nice aroma, and which ones smelled awful? Let me know in the comments!





