#aromatic compounds
What smells do you associate with “cleanness”? Is it the clean, dewy freshness after a thunderstorm (due to geosmin - Day 14)? Or is it that warm, enveloping smoothness of clean linen?
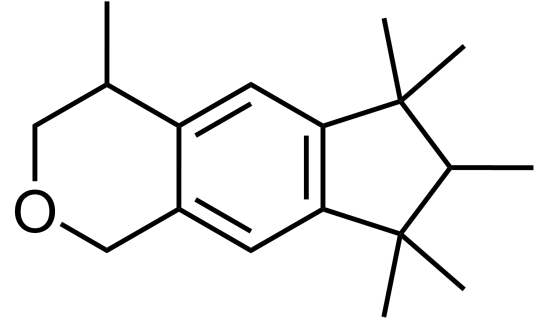
If you’re thinking about the latter, Galaxolide (C18H26O) is probably responsible for that. It belongs to a class of compounds called “musks”, which are extremely commonly used in fragrances, from functional (detergents and soaps) to fine (perfumes).
Musks used to be obtained from musk pods - a gland of musk deer - since ancient times, when they were discovered to not only have an aroma of their own, but also acted as a fixative for fragrances, which is to say that it prolonged the lasting power of fragrances that it was added to. Natural musk can also be obtained from civet cats and muskrats, amongst other animals.

However, in recent decades, the industry has turned to synthetic musks due to sustainability and ethical concerns regarding the harvesting of musk pods.
The first synthetic musks were discovered serendipitously in the late 19th century; a chemist, Albert Bauer, was trying to find a more explosive analogue to trinitrotoluene (TNT - Day 58) when he noticed that the products of his experiments had a pleasant musky odour. These “nitro musks”, as they were called, found widespread use due to their relatively low costs and similarity to natural musk. Chanel famously used nitro musks in many of their perfumes, including No. 5.
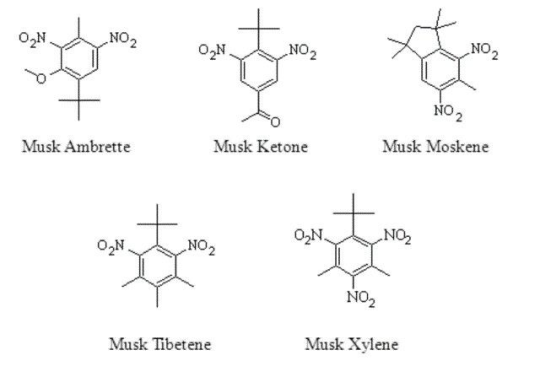
However, nitro musks were eventually suspected of neurotoxicity, and were phased out; only one nitro musk, musk ketone, is currently approved to be used in the fragrance industry.
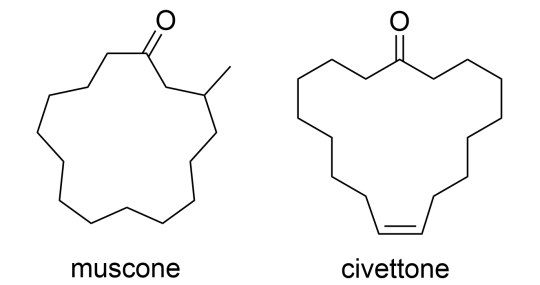
Other types of musks - macrocyclic musks, such as muscone and civettone, were isolated from natural musk from musk deer and civet cats. However, industrial synthesis proved too complicated and commercially unfeasible for the early 20th century.
The hunt for a new type of synthetic musk - one that was safe and cheap - was on again. In 1965, Galaxolide was first synthesised by International Flavors and Fragrances (IFF) in an attempt to make existing musks more chemically stable and hydrophobic, and was quickly utilised in many functional and fine fragrances due to its crisp, clean connotations; it was completely free of any animalic nuances, unlike molecules like muscone.
Why does the molecule have to be hydrophobic? Consider its purpose - when used in detergents, it would be a desirable quality to be able to adsorb onto fabric fibres and not be washed off by water. Its relatively large size meant that strong intermolecular forces of attractions would lead to low volatility too, thus allowing it to impart a long-lasting pleasant smell.

While Galaxolide was not the first polycyclic musk to be discovered, it is by far the most well-known one and is produced on a scale of 1,000 tons a year as of 2015.
However, with its ubiquity, its persistence in nature and its tendency to bioaccumulate has been brought under scrutiny. While no toxic effects to humans have been reported, it has still been a cause for concern as its effects on wildlife are not well understood.
And the search for new affordable, safe, and biodegradable musks continues…
My dear readers - what chemicals have you tried sniffing? Which ones had a nice aroma, and which ones smelled awful? Let me know in the comments!



Methamphetamine (C10H15N), also known as meth or crystal meth, is a colourless liquid at room temperature. It is more commonly encountered as the hydrochloride salt (C10H15N.HCl), which is a white solid under standard conditions. It is a central nervous system stimulant, and is used as a recreational drug.
Methamphetamine acts as an agonist at trace amine-associated receptor 1 (TAAR1), resulting in the release of cyclic adenosine monophosphate. This causes dopamine and noradrenaline transporters to reverse the movement of dopamine and noradrenaline through them; instead of taking them up from the synapse, it releases them from the cell. Furthermore, it inhibits monoamine oxidase (MAO), which normally breaks down dopamine and noradrenaline.

The resultant increase in dopamine and noradrenaline in the synapse causes the corresponding receptors on the postsynaptic membrane to be stimulated to a greater extent, resulting in feelings of euphoria, increased alertness, and a raised heart rate.
Methamphetamine, however, has a high risk of addiction. The high levels of dopamine and noradrenaline can result in tolerance by the body as the postsynaptic neuron reduces the number of receptors to modulate the stimulus. A protein called ΔFosB is also produced in the neurons, resulting in the increased transcription of certain genes, producing addictive behaviour.
As ΔFosB is degraded much more slowly than related proteins, it accumulates upon regular consumption of methamphetamine, resulting in increasing levels of addiction.

Methamphetamine also produces a range of side effects such as loss of appetite, dry skin, acne, insomnia, irregular heartbeat, psychosis, scratching of the skin, as well as loss of teeth. An overdose can also result in tremors, hyperthermia, cerebral haemorrhaging, kidney failure, circulatory collapse, coma, and death. (Below: before/after methamphetamine consumption)


It has been used as a treatment for attention-deficit hyperactivity disorder and obesity, albeit rarely due to its significant drawbacks compared to other existing treatments for these conditions. One of its isomers, levomethamphetamine (below left), is also used in nasal decongestant sprays as it results in vasoconstriction. Unlike its optical isomer, dextromethamphetamine (below right), it does not result in addiction and dependence.

Methamphetamine can be easily synthesised from the condensation of phenylacetone with methylamine, followed by reductive amination:

Note: This post is intended to examine the compound from a chemical/medical point of view for educational purposes, and does not endorse drug abuse in any way.


Omeprazole (C17H19N3O3S) is a drug used to treat acid reflux, stomach ulcers, and indigestion. Under standard conditions, it is a white powder that is sparingly soluble in water.
Omeprazole acts as an irreversible proton-pump inhibitor. It binds permanently to active H+/K+-ATPase systems found in the stomach lining, preventing H+ ions from being shuttled into the stomach. This causes a reduction in gastric acid production.

Being lipophilic, it is readily absorbed by the parietal cells of the stomach, where it undergoes an acid-catalysed rearrangement to form a sulfenic acid, which exists in equilibrium with the sulfenamide. The sulfenamide, which is the active form of the drug, can then react with a cysteine residue in the ATPase to form a covalent bond with it.

As active H+/K+-ATPase pumps are activated upon consumption of food, omeprazole should only be taken on an empty stomach, and food should only be taken 30-60 minutes after.
Proton-pump inhibitors should only be taken in appropriate doses when needed, as they have been shown to interfere with absorption of nutrients since gastric acid is essential for the digestion of food and release of nutrients.
Omeprazole can be synthesised via a multi-step process from 2,3,5-trimethylpyridine.

