#amines


Ammonia (NH3) is a binary compound of nitrogen and hydrogen. Commonly found in nature, it exists as a pungent, colourless gas under standard conditions, but is often sold as a solution in water. It is one of the most widely produced chemicals in the world, with 146 million tonnes being produced in 2016 alone.
Ammonia is a weak base, with a pKb of 4.75; it can reversibly react with water to produce ammonium and hydroxide ions.
NH3 + H2O ⇌ NH4+ + OH-
Due to the equilibrium shown above, solutions containing ammonia and ammonium ions are commonly used as buffer solutions, which resist pH changes upon addition of small amounts of acids or bases.
At the same time, ammonia can also act as an acid with very strong bases and reactive metals. For example, sodium metal reacts with ammonia to produce sodium amide, a strong base:
2 Na + 2 NH3 → 2 NaNH2 + H2
With its lone pair, ammonia can also coordinate to metal ions, resulting in colourful metal ion complexes, such as the deep blue tetraamminecopper(II) ion:

Ammonia is a versatile starting block for many chemical and fertiliser industries, as it offers a convenient way to introduce a nitrogen atom into a molecule. Being a nucleophile, it can participate in nucleophilic substitution and addition-elimination reactions, a useful trait that is exploited in many chemical syntheses. For example, the first step in the Strecker amino acid synthesis, which allowed chemists to synthesise amino acids for the first time instead of extracting it from organic material, involves the usage of ammonia to convert an aldehyde into an imine.

Ammonia is industrially produced by the Haber process, in which nitrogen is reacted with hydrogen under moderate temperature and high pressure in the presence of a catalyst to produce ammonia according to the following equation:
N2 + 3 H2 ⇌ 2 NH3
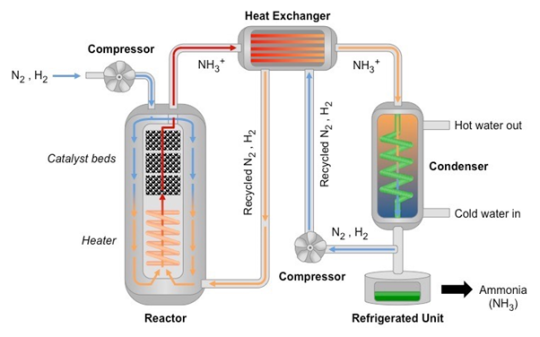
As the reaction is reversible, the reaction mixture is then cooled to condense the ammonia, and the leftover hydrogen and nitrogen is pumped back into the reactor to participate in the reaction again, thus maximising yield.
Ammonia is a metabolic waste from the digestion of proteins and other nitrogen-containing products, and is excreted through the urine. It is also produced from the decomposition of tissues.
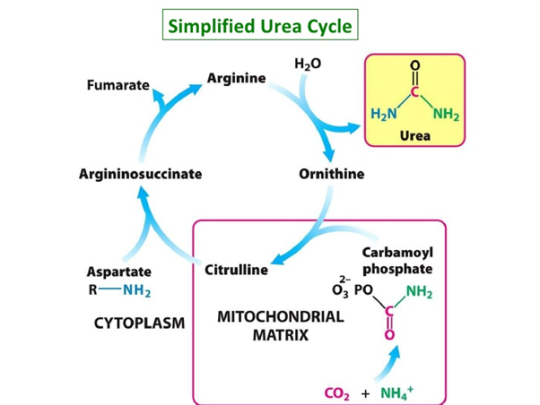
While ammonia is present in many tissues, it is metabolised into urea rapidly in the liver via the urea cycle, as urea is much less toxic and basic, and the buildup of ammonia can result in liver cirrhosis.


Methamphetamine (C10H15N), also known as meth or crystal meth, is a colourless liquid at room temperature. It is more commonly encountered as the hydrochloride salt (C10H15N.HCl), which is a white solid under standard conditions. It is a central nervous system stimulant, and is used as a recreational drug.
Methamphetamine acts as an agonist at trace amine-associated receptor 1 (TAAR1), resulting in the release of cyclic adenosine monophosphate. This causes dopamine and noradrenaline transporters to reverse the movement of dopamine and noradrenaline through them; instead of taking them up from the synapse, it releases them from the cell. Furthermore, it inhibits monoamine oxidase (MAO), which normally breaks down dopamine and noradrenaline.

The resultant increase in dopamine and noradrenaline in the synapse causes the corresponding receptors on the postsynaptic membrane to be stimulated to a greater extent, resulting in feelings of euphoria, increased alertness, and a raised heart rate.
Methamphetamine, however, has a high risk of addiction. The high levels of dopamine and noradrenaline can result in tolerance by the body as the postsynaptic neuron reduces the number of receptors to modulate the stimulus. A protein called ΔFosB is also produced in the neurons, resulting in the increased transcription of certain genes, producing addictive behaviour.
As ΔFosB is degraded much more slowly than related proteins, it accumulates upon regular consumption of methamphetamine, resulting in increasing levels of addiction.

Methamphetamine also produces a range of side effects such as loss of appetite, dry skin, acne, insomnia, irregular heartbeat, psychosis, scratching of the skin, as well as loss of teeth. An overdose can also result in tremors, hyperthermia, cerebral haemorrhaging, kidney failure, circulatory collapse, coma, and death. (Below: before/after methamphetamine consumption)


It has been used as a treatment for attention-deficit hyperactivity disorder and obesity, albeit rarely due to its significant drawbacks compared to other existing treatments for these conditions. One of its isomers, levomethamphetamine (below left), is also used in nasal decongestant sprays as it results in vasoconstriction. Unlike its optical isomer, dextromethamphetamine (below right), it does not result in addiction and dependence.

Methamphetamine can be easily synthesised from the condensation of phenylacetone with methylamine, followed by reductive amination:

Note: This post is intended to examine the compound from a chemical/medical point of view for educational purposes, and does not endorse drug abuse in any way.
