#pharmaceutical chemistry
MeetFlor Cobar, pharmaceutical chemist and drug development scientist
1) What do you do?
I am currently an MSc student in Pharmaceutical Modelling. I apply my theoretical and practical knowledge of advanced modelling techniques including computational chemistry, modelling of clinical and preclinical data, machine learning, bioinformatics and programming skills to various disciplines of drug discovery, development, and usage.
2) Where do you work?
I am currently doing my degree project in the field of computational medicinal chemistry at the Department of Pharmaceutical Chemistry, Drug Design and Development at Uppsala University in Sweden.
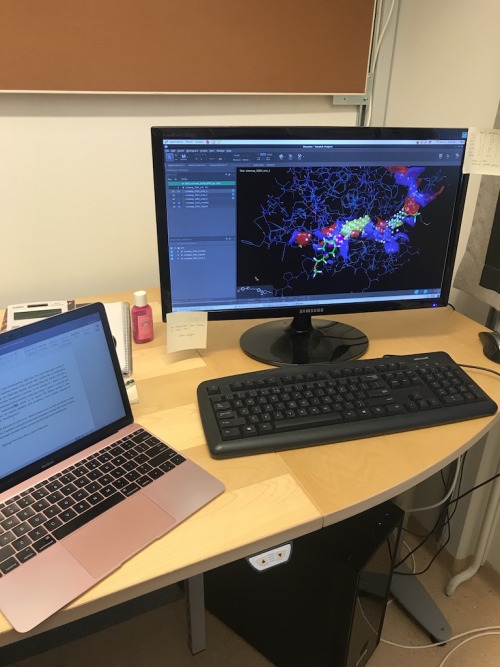
3) Tell us about the photos!
[Left:]A photo from my visit to Stockholm
[Right:] In the picture, I was mapping potential binding sites of MMP13 (matrix metalloproteinase 13) enzyme to evaluate druggability. It’s a small part of my project which is about optimization and evaluation of virtual screening protocols.
4) Tell us about your academic career path so far.
I finished BS Pharmacy in CEU Manila and continued to obtain a Doctor of Pharmacy in CEU Makati. Immediately after I finished PharmD, I taught in CEU Manila and Makati for 2 years. I applied for a scholarship in Uppsala University and I was awarded with IPK Uppsala University Scholarship.
5) Anything else you’d like to share
In silico (or performed in computer or via computer simulation) methodologies in the field of pharmaceutical sciences has not been accepted or practiced yet in the Philippines (to my knowledge). These techniques will help us make smart decisions of identifying compounds/molecules that are needed to be prioritized and tested, thus saving a lot of time and money in drug discovery and development. As an academician and a researcher, I hope that in silico methods will be practiced in pharmaceutical research in the Philippines as it is becoming the norm in EU, USA and in Japan.
Post link


Methamphetamine (C10H15N), also known as meth or crystal meth, is a colourless liquid at room temperature. It is more commonly encountered as the hydrochloride salt (C10H15N.HCl), which is a white solid under standard conditions. It is a central nervous system stimulant, and is used as a recreational drug.
Methamphetamine acts as an agonist at trace amine-associated receptor 1 (TAAR1), resulting in the release of cyclic adenosine monophosphate. This causes dopamine and noradrenaline transporters to reverse the movement of dopamine and noradrenaline through them; instead of taking them up from the synapse, it releases them from the cell. Furthermore, it inhibits monoamine oxidase (MAO), which normally breaks down dopamine and noradrenaline.

The resultant increase in dopamine and noradrenaline in the synapse causes the corresponding receptors on the postsynaptic membrane to be stimulated to a greater extent, resulting in feelings of euphoria, increased alertness, and a raised heart rate.
Methamphetamine, however, has a high risk of addiction. The high levels of dopamine and noradrenaline can result in tolerance by the body as the postsynaptic neuron reduces the number of receptors to modulate the stimulus. A protein called ΔFosB is also produced in the neurons, resulting in the increased transcription of certain genes, producing addictive behaviour.
As ΔFosB is degraded much more slowly than related proteins, it accumulates upon regular consumption of methamphetamine, resulting in increasing levels of addiction.

Methamphetamine also produces a range of side effects such as loss of appetite, dry skin, acne, insomnia, irregular heartbeat, psychosis, scratching of the skin, as well as loss of teeth. An overdose can also result in tremors, hyperthermia, cerebral haemorrhaging, kidney failure, circulatory collapse, coma, and death. (Below: before/after methamphetamine consumption)


It has been used as a treatment for attention-deficit hyperactivity disorder and obesity, albeit rarely due to its significant drawbacks compared to other existing treatments for these conditions. One of its isomers, levomethamphetamine (below left), is also used in nasal decongestant sprays as it results in vasoconstriction. Unlike its optical isomer, dextromethamphetamine (below right), it does not result in addiction and dependence.

Methamphetamine can be easily synthesised from the condensation of phenylacetone with methylamine, followed by reductive amination:

Note: This post is intended to examine the compound from a chemical/medical point of view for educational purposes, and does not endorse drug abuse in any way.


Omeprazole (C17H19N3O3S) is a drug used to treat acid reflux, stomach ulcers, and indigestion. Under standard conditions, it is a white powder that is sparingly soluble in water.
Omeprazole acts as an irreversible proton-pump inhibitor. It binds permanently to active H+/K+-ATPase systems found in the stomach lining, preventing H+ ions from being shuttled into the stomach. This causes a reduction in gastric acid production.

Being lipophilic, it is readily absorbed by the parietal cells of the stomach, where it undergoes an acid-catalysed rearrangement to form a sulfenic acid, which exists in equilibrium with the sulfenamide. The sulfenamide, which is the active form of the drug, can then react with a cysteine residue in the ATPase to form a covalent bond with it.

As active H+/K+-ATPase pumps are activated upon consumption of food, omeprazole should only be taken on an empty stomach, and food should only be taken 30-60 minutes after.
Proton-pump inhibitors should only be taken in appropriate doses when needed, as they have been shown to interfere with absorption of nutrients since gastric acid is essential for the digestion of food and release of nutrients.
Omeprazole can be synthesised via a multi-step process from 2,3,5-trimethylpyridine.