#ammonia
A robust material for the uptake and storage of ammonia at densities that come close to that of the liquefied gas
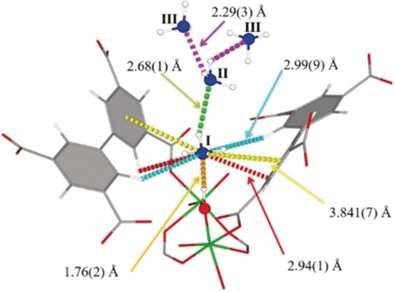
Handling, storing, and shipping of ammonia requires costly equipment and special precautions because of its inherent corrosiveness and toxicity. Scientists in Manchester, UK, have found that a metal–organic framework, MFM-300(Al), a porous solid, not only effectively filters harmful nitrogen dioxide gas, but it also has outstanding capabilities for ammonia storage. As detailed in the journal Angewandte Chemie, reversible uptake and release of ammonia proceeds by a unique sorption mode.
Ammonia is an essential nitrogen source for plants and it is a basic chemical. This indispensable chemical, which is manufactured on a large scale from atmospheric nitrogen and hydrogen, has been called “bread from air”. But how should this resource be stored and handled? The gaseous or liquefied form is corrosive and toxic. Storing and shipping under pressure or at low temperatures is costly and energy-consuming. Adsorption in porous solids, such as zeolites or metal–organic frameworks—a strategy currently being tested extensively in hydrogen storage—could be an interesting option.
The robust metal–organic framework MFM-300(Al) has been shown to be a potent filter for nitrogen dioxide, which is a harmful pollutant in air. Martin Schröder and his colleagues at the University of Manchester, UK, have now scrutinized MFM-300(Al) for its ability to take up ammonia. They discovered that it could take up gaseous ammonia up to a density that comes close to that of liquid ammonia under ambient conditions. At around zero degrees Celsius it even surpassed this density.
Post link
Light makes Rice U. catalyst more effective: Halas lab details plasmonic effect that allows catalyst to work at lower energy
Rice University nanoscientists have demonstrated a new catalyst that can convert ammonia into hydrogen fuel at ambient pressure using only light energy, mainly due to a plasmonic effect that makes the catalyst more efficient.
[…]
A study from Rice’s Laboratory for Nanophotonics (LANP) in this week’s issue of Science describes the new catalytic nanoparticles, which are made mostly of copper with trace amounts of ruthenium metal. Tests showed the catalyst benefited from a light-induced electronic process that significantly lowered the “activation barrier,” or minimum energy needed, for the ruthenium to break apart ammonia molecules.
The research comes as governments and industry are investing billions of dollars to develop infrastructure and markets for carbon-free liquid ammonia fuel that will not contribute to greenhouse warming. But the researchers say the plasmonic effect could have implications beyond the “ammonia economy.”
Post link
Producing ammonia through electrochemical processes could reduce carbon dioxide emissions
Ammonia is commonly used in fertilizer because it has the highest nitrogen content of commercial fertilizers, making it essential for crop production. However, two carbon dioxide molecules are made for every molecule of ammonia produced, contributing to excess carbon dioxide in the atmosphere.
A team from the Artie McFerrin Department of Chemical Engineering at Texas A&M University consisting of Dr. Abdoulaye Djire, assistant professor, and graduate student Denis Johnson, has furthered a method to produce ammonia through electrochemical processes, helping to reduce carbon emissions. This research aims to replace the Haber-Bosch thermochemical process with an electrochemical process that is more sustainable and safer for the environment.
The researchers recently published their findings in Scientific Reports.
Since the early 1900s, the Haber-Bosch process has been used to produce ammonia. This process works by reacting atmospheric nitrogen with hydrogen gas. A downside of the Haber-Bosch process is that it requires high pressure and high temperature, leaving a large energy footprint. The method also requires hydrogen feedstock, which is derived from nonrenewable resources. It is not sustainable and has negative implications on the environment, expediting the need for new and environmentally friendly processes.
Post link
New process aims to strip ammonia from wastewater
A dash of ruthenium atoms on a mesh of copper nanowires could be one step toward a revolution in the global ammonia industry that also helps the environment.
Collaborators at Rice University’s George R. Brown School of Engineering, Arizona State University and Pacific Northwest National Laboratory developed the high-performance catalyst that can, with near 100% efficiency, pull ammonia and solid ammonia—aka fertilizer—from low levels of nitrates that are widespread in industrial wastewater and polluted groundwater.
A study led by Rice chemical and biomolecular engineer Haotian Wang shows the process converts nitrate levels of 2,000 parts per million into ammonia, followed by an efficient gas stripping process for ammonia product collection. The remaining nitrogen contents after these treatments can be brought down to “drinkable” levels as defined by the World Health Organization.
“We fulfilled a complete water denitrification process,” said graduate student Feng-Yang Chen. “With further water treatment on other contaminants, we can potentially turn industrial wastewater back to drinking water.”
Post link
The Ultimate Guide to Potion Bases
We all spend so much time thinking about the correspondences of the actual ingredients that go into our potions that we often forget to think about what the potion base represents! (At least I do.) It would be nice to have a list of all the various liquids that can be used in place of water. Naturally, I can’t think of everything but I think this is a pretty good starting point! What else can be used? Eventually, at some point down the road, I will compile all these thoughts into a book on potion making and want to include this! Keep in mind that these are my own correspondences. Let me know if you disagree or if you’d change anything up! Let’s see how big we can make this list. Also, I should probably note that not all of these liquids can be ingested. (Obviously.)
The List
Vinegar: Used for cleansing and purification potions.
Lemon Juice: Used in hexing, cursing, or revenge potions.
Cranberry Juice: Used in love potions.
Apple Juice: Used in healing, knowledge, and youth potions.
Ammonia: Used in banishing, cursing, purification, and protection
Red Wine: Love potions and potions dealing with death and the afterlife.
White Wine: Used in platonic love potions as well as success brews.
Rum: Used in potions involving spirit work.
Whisky: Another good base for potion work.
Vodka: A good base for work involving rapid banishing.
Laundry detergent: Good for cleansing potions.
Oils: Used to speed up a process.
Molasses: Used in potions intended to slow a situation down.
Rubbing Alcohol: Another good base for cleansing and purification.
Hydrogen Peroxide: Used in healing potions.
Milk: Used in potions to promote sleep and peace.
Sour Milk: Used to cause nightmares or in potions designed to torment.
Orange Juice: For potions of solar importance, healing, success.
Soda Water: Used in potions designed to encourage laughter and giddiness.
Ginger Ale: Used in health or healing potions.
Olive Juice: Used in peace potions.
Honey: Used in potions to sweeten up another’s disposition.
Syrup: Used in abundance and prosperity potions.
Beer: Used in potions intended to induce slumber.
Clam Juice: Used in aphrodisiacs.
Cough Syrup: Used in healing potions and to make someone ‘cough it up.’
Soy Sauce: Used in protection potions. (Thanks Lexa Rosean for this one!)
Pineapple Juice: Used in abundance potions and fidelity potions.
Coconut Milk: Used in spiritual and magical cleansing potions.
Ice: Solid first, then melted for transformation potions.
Coffee: Really, a potion in and of itself in my book.
Vanilla Extract: In small amounts, used in passion potions.
Witch Hazel: Used in communication and cleansing potions.What else can you all think of?
Post link


Ammonia (NH3) is a binary compound of nitrogen and hydrogen. Commonly found in nature, it exists as a pungent, colourless gas under standard conditions, but is often sold as a solution in water. It is one of the most widely produced chemicals in the world, with 146 million tonnes being produced in 2016 alone.
Ammonia is a weak base, with a pKb of 4.75; it can reversibly react with water to produce ammonium and hydroxide ions.
NH3 + H2O ⇌ NH4+ + OH-
Due to the equilibrium shown above, solutions containing ammonia and ammonium ions are commonly used as buffer solutions, which resist pH changes upon addition of small amounts of acids or bases.
At the same time, ammonia can also act as an acid with very strong bases and reactive metals. For example, sodium metal reacts with ammonia to produce sodium amide, a strong base:
2 Na + 2 NH3 → 2 NaNH2 + H2
With its lone pair, ammonia can also coordinate to metal ions, resulting in colourful metal ion complexes, such as the deep blue tetraamminecopper(II) ion:

Ammonia is a versatile starting block for many chemical and fertiliser industries, as it offers a convenient way to introduce a nitrogen atom into a molecule. Being a nucleophile, it can participate in nucleophilic substitution and addition-elimination reactions, a useful trait that is exploited in many chemical syntheses. For example, the first step in the Strecker amino acid synthesis, which allowed chemists to synthesise amino acids for the first time instead of extracting it from organic material, involves the usage of ammonia to convert an aldehyde into an imine.

Ammonia is industrially produced by the Haber process, in which nitrogen is reacted with hydrogen under moderate temperature and high pressure in the presence of a catalyst to produce ammonia according to the following equation:
N2 + 3 H2 ⇌ 2 NH3
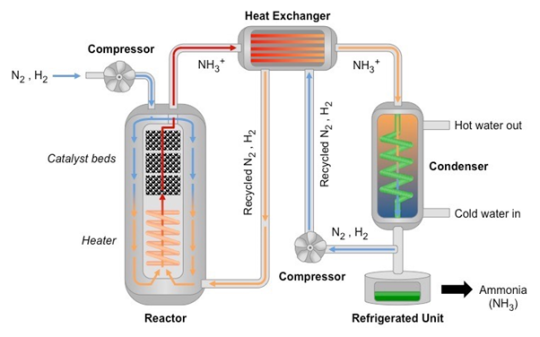
As the reaction is reversible, the reaction mixture is then cooled to condense the ammonia, and the leftover hydrogen and nitrogen is pumped back into the reactor to participate in the reaction again, thus maximising yield.
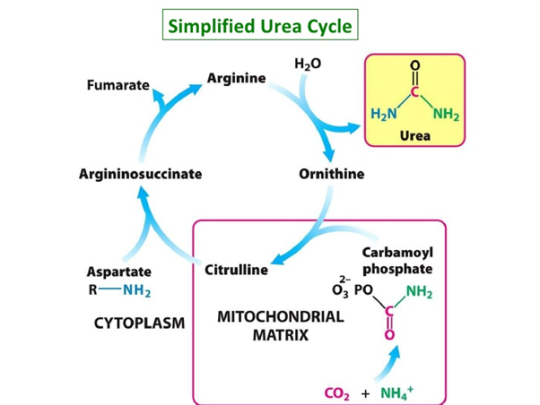
Ammonia is a metabolic waste from the digestion of proteins and other nitrogen-containing products, and is excreted through the urine. It is also produced from the decomposition of tissues.
While ammonia is present in many tissues, it is metabolised into urea rapidly in the liver via the urea cycle, as urea is much less toxic and basic, and the buildup of ammonia can result in liver cirrhosis.
ClassNK issues Approval in Principle (AiP) for Ammonia-ready VLGC developed by Mitsubishi Shipbuilding
ClassNK issues Approval in Principle (AiP) for Ammonia-ready VLGC developed by Mitsubishi Shipbuilding