#electrical conductivity
Team develops new material for wearable devices able to restore conductivity
The research team of researcher Hyunseon Seo and senior researcher Dr. Donghee Son of the Korea Institute of Science and Technology’s Biomedical Research Institute, postdoctoral candidate Dr. Jiheong Kang and Professor Zhenan Bao of Stanford University (chemical engineering) announced a new material with high stretchability and high electrical conductivity, with the ability to self-heal even after being subjected to severe mechanical strain. The material could have application in wearable electronic devices.
Prior to this study, Dr. Donghee Son, Dr. Jiheong Kang, and Prof. Zhenan Bao developed a polymer material that is highly elastic, can self-heal without the help of external stimuli even when exposed to water or sweat, and has a mechanical strength similar to that of human skin, making it comfortable to wear for long periods of time.
In its most recent study, the KIST-Stanford research team developed the new material, which can be used as an interconnect, as it has the same properties as existing wearable materials and high levels of electrical conductivity and stretchability, characteristics which allow the stable transmission of electricity and data from the human body to electronic devices.
Post link
From these seeds, new touchscreens and solar cells may grow. Duke University chemists are working with copper oxide nanoparticles–each in the pic above is less than a micron wide–to grow copper nanowires. The process could one day allow transparent conductive films made of copper nanowires to supplement or replace the more expensive material now used in touchscreens and photovoltaic solar panels.
“The fact that Cu [copper] is only 6 percent less conductive than the most conductive element, Ag [silver], and yet is 1,000 times more abundant, makes it a particularly attractive element from which to grow nanowires for a diverse range of applications that require high electrical conductivity,” wrote the authors of study published in the journal Small.
When placed in the right solution, the octahedral cuprous oxide (Cu2O) seeds shown above sprout nanowires within minutes. The Duke team’s work is continuing efforts to control the length of nanowire growth to improve their performance in different applications.
Post link
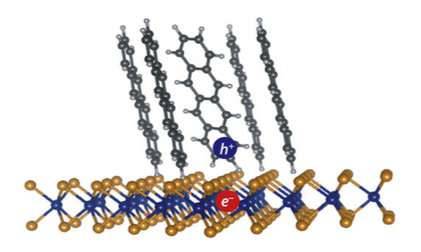
Fast-moving electrons create current in organic solar cells
Researchers at Purdue University have identified the mechanism that allows organic solar cells to create a charge, solving a longstanding puzzle in physics, according to a paper published Friday (Jan. 12) in the journal Science Advances.
Organic solar cells are built with soft molecules, while inorganic solar cells, often silicon-based, are built with more rigid materials. Silicon cells currently dominate the industry, but they’re expensive and stiff, while organic cells have the potential to be light, flexible and cheap. The drawback is that creating an electric current in organic cells is much more difficult.
To create an electrical current, two particles, one with a negative charge (electron) and one with a positive charge (electron-hole), must separate despite being bound tightly together. These two particles, which together form an exciton, usually require a manmade interface to separate them. The interface draws the electron through an electron acceptor and leaves the hole behind. Even with the interface in place, the electron and hole are still attracted to each other – there’s another mechanism that helps them separate.
“We discovered that this type of electron-hole interface is not one single static state. The electron and the hole can be far apart or close together, and the farther apart they are, the more likely they are to separate,” said Libai Huang, an assistant professor of chemistry in Purdue’s College of Science, who led the research. “When they’re far apart, they’re actually very mobile, and they can move pretty fast. We think that this kind of fast motion between the positive and negative charge is what’s driving separation at these interfaces.”
Post link