#organic materials
Australian shrub contains new class of organic compound
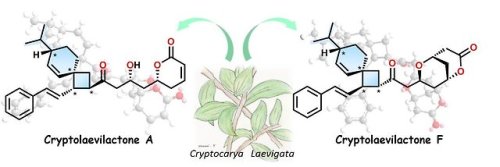
The botanical world can be an exciting place for chemists. Plant species produce a beautiful array of organic molecules with complex structures, often of great practical use. Indeed, this is a realm where new discoveries are still being made. Recently, a Japanese-led research team discovered an entirely new structural class in compounds from a jungle-dwelling shrub.
The glossy or red-fruited laurel (binomial name: Cryptocarya laevigata) inhabits the rainforests of eastern Australia. Little was known about the chemical makeup of this tall shrub until the team, led by Kanazawa University, analyzed an extract of its twigs and leaves. The plant’s essential oil was found to contain a family of six new compounds, the structural analysis of which revealed some surprises.
As reported in Organic Letters, NMR experiments showed that at the center of the compounds lay a peculiar, nine-membered carbon cycle known as a spiro-nonene. This structure consists of two rings of carbon atoms—one containing six atoms, the other four—linked by a single “pinch point” atom that is a part of both rings. This motif had never been seen before in any natural product.
Post link
Supercomputer predicts optical and thermal properties of complex hybrid materials
Materials scientists at Duke University computationally predicted the electrical and optical properties of semiconductors made from extended organic molecules sandwiched by inorganic structures.
These types of so-called layered “hybrid organic-inorganic perovskites"—or HOIPs—are popular targets for light-based devices such as solar cells and light-emitting diodes (LEDs). The ability to build accurate models of these materials atom-by-atom will allow researchers to explore new material designs for next-generation devices.
The results appeared online on October 4 in Physical Review Letters.
"Ideally we would like to be able to manipulate the organic and inorganic components of these types of materials independently and create semiconductors with new, predictable properties,” said David Mitzi, the Simon Family Professor of Mechanical Engineering and Materials Science at Duke. “This study shows that we are able to match and explain the experimental properties of these materials through complex supercomputer simulations, which is quite exciting.”
Post link
New Way to Power Up Nanomaterials to Create Better Solar Cells and LEDs
UCLA materials scientists and colleagues have discovered that perovskites, a class of promising materials that could be used for low-cost, high-performance solar cells and LEDs, have a previously unutilized molecular component that can further tune the electronic property of perovskites.
Named after Russian mineralogist Lev Perovski, perovskite materials have a crystal-lattice structure of inorganic molecules like that of ceramics, along with organic molecules that are interlaced throughout. Up to now, these organic molecules appeared to only serve a structural function and could not directly contribute to perovskites’ electronic performance.
Led by UCLA, a new study shows that when the organic molecules are designed properly, they not only can maintain the crystal lattice structure, but also contribute to the materials’ electronic properties. This discovery opens up new possibilities to improve the design of materials that will lead to better solar cells and LEDs. The study detailing the research was recently published in Science.
Post link
Solid + solid = liquid?
Can a mix of solids form a liquid? In a “deep eutectic system” (DES), yes. In these cases—which includes some mixtures of sugars, amino acids, and organic acids—compounds that are typically solid at room temperature lower each other’s melting points when combined in some ratios. As a result, they melt. Starting at the left, this picture shows two natural compounds, menthol and lauric acid. Combined in a certain molar fraction as they were heated and stirred, the compounds formed the eutectic liquid in the flask on the right. After the liquid is formed, it remains stable at room temperature. This is also what happens with honey, a naturally occurring DES that is a viscous liquid resulting from the mixture of various sugars. Project Des.Solve, funded by the European Research Council, aims to extend knowledge of these systems, focusing on their characterization and boosting application to fields including extraction, biocatalysis, green chemistry, and biomedical science. — Craig Bettenhausen
Submitted by Liane Meneses and Luísa Pereira/Project Des.Solve
Do science. Take pictures. Win money. Enter our photo contest here.
Post link
Organic ferromagnetism: Trapping spins in glassy state
An international team of researchers, affiliated UNIST has introduced an exiting new organic network structure that shows pure organic ferromagnetic property at room temperature. As described in the CHEM journal this pure organic material exhibits ferromagnetism from pure p-TCNQ without any metal contamination.
This breakthrough has been led by by Professor Jong-Beom Baek and his research team in the School of the Energy and Chemical Engineering at UNIST. In the study, the research team has synthesized a network structure from the self polymerization of tetracyanoquinodimethane (TCNQ) monomer. The designed organic network structure generates stable neutral radicals.
For over two decades, there has been widespread scepticism around claims of organic plastic ferromagnetism, mostly due to contamination by transition metals. Extensive effort has been devoted to developing magnets in purely organic compounds based on free radicals, driven by both scientific curiosity and the potential applications of a ‘plastic magnet’. Excluding the contamination issues and realizing magnetic properties from pure organic plastics must occur to revive the quest for plastic magnetism.
Post link
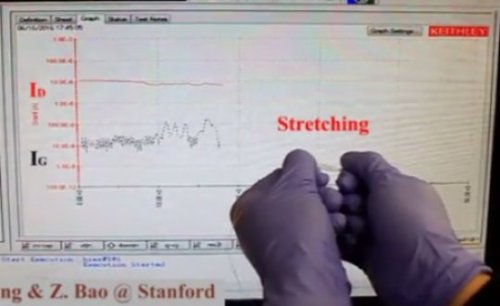
A flexible transistor that conforms to skin
Researchers have created a stretchy transistor that can be elongated to twice its length with only minimal changes in its conductivity. The development is a valuable advancement for the field of wearable electronics. To date, it has been difficult to design a transistor using inherently stretchable materials that maintains its conductivity upon being stretched.
Here, Jie Xu and devise a clever and scalable way to confine organic conductors inside a rubbery polymer to create stretchy transistors. They took a semiconducting polymer, called DPPT-TT, and confined it inside another polymer, SEBS, which has elastic properties.
As the two polymers don’t like to mix with each other, the DPPT-TT forms thin bundles within the SEBS matrix. Testing and analysis of this new combination reveal that it works as an effective transistor, even as it is repeatedly stretched up to 100% of its length. While the material demonstrated a normal conductivity of 0.59 cm2/Vs on average, this dropped only slightly to 0.55 cm2/Vs when being stretched to twice its length.
Post link
Rubber material holds key to long-lasting, safer EV batteries
For electric vehicles (EVs) to become mainstream, they need cost-effective, safer, longer-lasting batteries that won’t explode during use or harm the environment. Researchers at the Georgia Institute of Technology may have found a promising alternative to conventional lithium-ion batteries made from a common material: rubber.
Elastomers, or synthetic rubbers, are widely used in consumer products and advanced technologies such as wearable electronics and soft robotics because of their superior mechanical properties. The researchers found that the material, when formulated into a 3D structure, acted as a superhighway for fast lithium-ion transport with superior mechanical toughness, resulting in longer charging batteries that can go farther. The research, conducted in collaboration with the Korea Advanced Institute of Science and Technology, was published Wednesday in the journal Nature.
Inconventional lithium-ion batteries, ions are moved by a liquid electrolyte. However, the battery is inherently unstable: even the slightest damage can leak into the electrolyte, leading to explosion or fire. The safety issues have forced the industry to look at solid-state batteries, which can be made using inorganic ceramic material or organic polymers.
Post link
Making the internet of things possible with a new breed of ‘memristors’
Easily printable, organic thin films can retain data for more than 10 years without power, work with low voltages – and become the building block of future computers that mimic the human brain
The internet of things is coming, that much we know. But still it won’t; not until we have components and chips that can handle the explosion of data that comes with IoT. In 2020, there will already be 50 billion industrial internet sensors in place all around us. A single autonomous device – a smart watch, a cleaning robot, or a driverless car – can produce gigabytes of data each day, whereas an airbus may have over 10,000 sensors in one wing alone.
Two hurdles need to be overcome. First, current transistors in computer chips must be miniaturized to the size of only few nanometres; the problem is they won’t work anymore then. Second, analysing and storing unprecedented amounts of data will require equally huge amounts of energy. Sayani Majumdar, Academy Fellow at Aalto University, along with her colleagues, is designing technology to tackle both issues.
Majumdar has with her colleagues designed and fabricated the basic building blocks of future components in what are called “neuromorphic” computers inspired by the human brain. It’s a field of research on which the largest ICT companies in the world and also the EU are investing heavily. Still, no one has yet come up with a nano-scale hardware architecture that could be scaled to industrial manufacture and use.
Post link
Fast-moving electrons create current in organic solar cells
Researchers at Purdue University have identified the mechanism that allows organic solar cells to create a charge, solving a longstanding puzzle in physics, according to a paper published Friday (Jan. 12) in the journal Science Advances.
Organic solar cells are built with soft molecules, while inorganic solar cells, often silicon-based, are built with more rigid materials. Silicon cells currently dominate the industry, but they’re expensive and stiff, while organic cells have the potential to be light, flexible and cheap. The drawback is that creating an electric current in organic cells is much more difficult.
To create an electrical current, two particles, one with a negative charge (electron) and one with a positive charge (electron-hole), must separate despite being bound tightly together. These two particles, which together form an exciton, usually require a manmade interface to separate them. The interface draws the electron through an electron acceptor and leaves the hole behind. Even with the interface in place, the electron and hole are still attracted to each other – there’s another mechanism that helps them separate.
“We discovered that this type of electron-hole interface is not one single static state. The electron and the hole can be far apart or close together, and the farther apart they are, the more likely they are to separate,” said Libai Huang, an assistant professor of chemistry in Purdue’s College of Science, who led the research. “When they’re far apart, they’re actually very mobile, and they can move pretty fast. We think that this kind of fast motion between the positive and negative charge is what’s driving separation at these interfaces.”
Post link
A major step forward in organic electronics
Researchers at the Laboratory of Organic Electronics, Linköping University, have developed the world’s first complementary electrochemical logic circuits that can function stably for long periods in water. This is a highly significant breakthrough in the development of bioelectronics.
The first printable organic electrochemical transistors were presented by researchers at LiU as early as 2002, and research since then has progressed rapidly. Several organic electronic components, such as light-emitting diodes and electrochromic displays, are already commercially available.
The most commonly used material is PEDOT:PSS, which is a p-type material in which the charge carriers are holes. In order to construct effective electron components, a complementary material, n-type, is required, in which the charge carriers are electrons. It has been difficult to find a sufficiently stable polymer material that can operate in water media and in which the long polymer chains can sustain high current when the material is doped.
In an article in Advanced Materials, Simone Fabiano and colleagues have presented results from an n-type conducting material in which the ladder-type structure of the polymer backbone favours ambient stability and high current when doped. One example is BBL, poly(benzimidazobenzophenanthroline), a material often used in solar cell research.
Post link
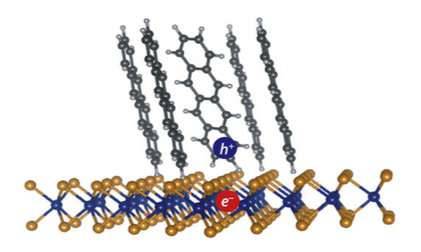
Hybrid nanomaterials bristle with potential
By combining multiple nanomaterials into a single structure, scientists can create hybrid materials that incorporate the best properties of each component and outperform any single substance. A controlled method for making triple-layered hollow nanostructures has now been developed at KAUST. The hybrid structures consist of a conductive organic core sandwiched between layers of electrocatalytically active metals: their potential uses range from better battery electrodes to renewable fuel production.
Although several methods exist to create two-layer materials, making three-layered structures has proven much more difficult, says Peng Wang from the Water Desalination and Reuse Center who co-led the current research with Professor Yu Han, member of the Advanced Membranes and Porous Materials Center at KAUST. The researchers developed a new, dual-template approach, explains Sifei Zhuo, a postdoctoral member of Wang’s team.
The researchers grew their hybrid nanomaterial directly on carbon paper—a mat of electrically conductive carbon fibers. They first produced a bristling forest of nickel cobalt hydroxyl carbonate (NiCoHC) nanowires onto the surface of each carbon fiber (image 1). Each tiny inorganic bristle was coated with an organic layer called hydrogen substituted graphdiyne (HsGDY) (image 2).
Post link
Gather&See curate collections from the best in sustainable fashion from around the world, showcasing sustainable fashion in a fresh and innovative way.
Post link
Annie + Jade produce a stylish ethical collection with a casual sophistication. The timeless aesthetic embodies the simple elegance of a high-end resort collection, with simple clean lines, and a strong silhouette.
Post link
Launched in 2009, Outsider is based around the premis that “Ethical fashion should look just like fashion”.
Post link