#electrocatalysts
Novel use of NMR sheds light on easy-to-make electropolymerized catalysts
In the world of catalytic reactions, polymers created through electropolymerization are attracting renewed attention. A group of Chinese researchers recently provided the first detailed characterization of the electrochemical properties of polyaniline and polyaspartic acid (PASP) thin films. In AIP Advances, the team used a wide range of tests to characterize the polymers, especially their capacity for catalyzing the oxidation of popularly used materials, hydroquinone and catechol.
This new paper marks one of the first pairings of standard electrochemical tests with nuclear magnetic resonance (NMR) analysis in such an application. “Because these materials can be easily prepared in an electric field and are cost-effective and environmentally friendly, we think they have the potential to be widely used,” said Shuo-Hui Cao, an author on the paper.
Although PASP has shown excellent electrocatalytic responses to biological molecules, newer areas of inquiry have explored the material’s ability to lower the oxidational potential in oxidation-reduction reactions. Reducing the oxidation potential is key for finding further uses for two materials used extensively as raw materials and synthetic intermediates in pharmaceuticals, hydroquinone and catechol.
Post link
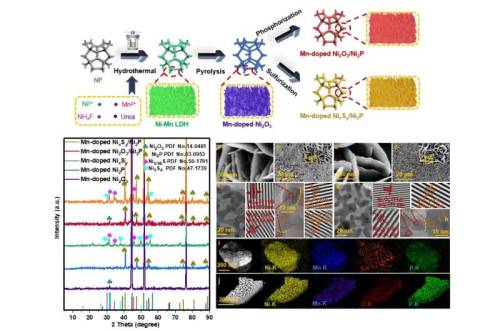
Three dimensional Mn-doped nanosheets as efficient electrocatalysts for alkaline water splitting
Hydrogen has attracted extensive attention from academia and industry as an energy source due to its intrinsic environmental compatibility, abundance, and high energy density (120 MJ kg−1). Electrocatalytic water splitting is an environmentally friendly route to produce hydrogen, especially when the electricity is from renewable sources that minimize carbon dioxide emissions throughout the process.
Theoxygen evolution reaction (OER) on the anode and hydrogen evolution reaction (HER) on the cathode are two half-reactions in electrocatalytic water splitting. Pt- and Ru/Ir-based compounds are the best-known high-performance noble metal electrocatalysts for HER and OER, respectively. However, the scarcity and high cost of such noble metals hamper their application in water electrolysis. Therefore, with global prospects, it is essential to develop earth-abundant non-noble metal electrocatalysts for next-generation water splitting technologies. Recently, Ni-based electrocatalysts have been confirmed to be effective for boosting electrocatalytic water splitting, but their performances are not high enough for large-scale hydrogen production.
A team in China has successfully fabricated Mn-doped Ni2O3/Ni2P and Mn-doped NixSy/Ni2P through facile hydrothermal reaction and subsequently phosphorization and sulfurization method.
Post link
A treasure map for the realm of electrocatalysts
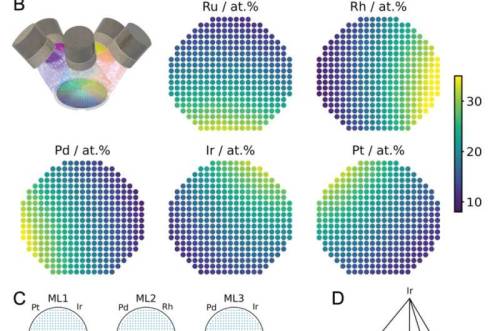
High entropy alloys (HEAs) are chemically complex materials made up of mixtures of five or more elements. What’s interesting about them is that they offer completely new possibilities for the development of electrocatalysts. Such catalysts are urgently needed to make energy conversion processes more efficient, for example for the production and use of green hydrogen. “The problem with HEAs is that, in principle, millions of high-entropy systems are possible and each system involves tens of thousands of different compositions,” explains Professor Alfred Ludwig, who heads the Materials Discovery and Interfaces Chair at RUB. It is almost impossible to tackle such complexity using conventional methods and traditional high-throughput procedures.
Five sources, six constellations
The researchers describe a new method in their paper that should help to find promising high entropy alloys for electrocatalysis. In the first step, the team developed a way to produce as many potential compositions as possible. For this purpose, they used a sputtering system that simultaneously applies the five base materials to a carrier. “You can imagine this as five spray cans directed at one point on the target,” explains RUB researcher Dr. Lars Banko. This produces a very specific composition of the five source materials on each point of the carrier, so-called materials libraries. Since this composition is also affected by the position of the sources of the source materials, the research team modified them in the experiment. The materials libraries from the manufacturing processes with six different constellations of the sources were subsequently characterized using high-throughput measurements.
Post link