#electrochemical
Novel use of NMR sheds light on easy-to-make electropolymerized catalysts
In the world of catalytic reactions, polymers created through electropolymerization are attracting renewed attention. A group of Chinese researchers recently provided the first detailed characterization of the electrochemical properties of polyaniline and polyaspartic acid (PASP) thin films. In AIP Advances, the team used a wide range of tests to characterize the polymers, especially their capacity for catalyzing the oxidation of popularly used materials, hydroquinone and catechol.
This new paper marks one of the first pairings of standard electrochemical tests with nuclear magnetic resonance (NMR) analysis in such an application. “Because these materials can be easily prepared in an electric field and are cost-effective and environmentally friendly, we think they have the potential to be widely used,” said Shuo-Hui Cao, an author on the paper.
Although PASP has shown excellent electrocatalytic responses to biological molecules, newer areas of inquiry have explored the material’s ability to lower the oxidational potential in oxidation-reduction reactions. Reducing the oxidation potential is key for finding further uses for two materials used extensively as raw materials and synthetic intermediates in pharmaceuticals, hydroquinone and catechol.
Post link
Disorder can stabilize batteries
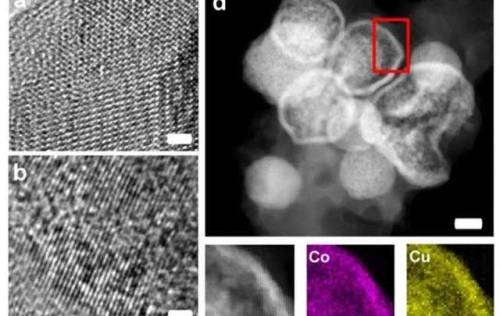
Novel materials can considerably improve storage capacity and cycling stability of rechargeable batteries. Among these materials are high-entropy oxides (HEO), whose stability results from a disordered distribution of the elements. With HEO, electrochemical properties can be tailored, as was found by scientists of the team of nanotechnology expert Horst Hahn at Karlsruhe Institute of Technology (KIT). The researchers report their findings in the journal Nature Communications.
Sustainable energy supply requires reliable storage systems. Demand for rechargeable electrochemical energy storage devices for both stationary and mobile applications has increased rapidly in the past years and is expected to continue to grow in the future. Among the most important properties of batteries are their storage capacity and their cycling stability, i.e. the number of possible charging and discharging processes without any loss of capacity. Thanks to its high stability, an entirely new class of materials called high-entropy oxides (HEO) is expected to result in major improvements. Moreover, electrochemical properties of HEO can be customized by varying their compositions. For the first time, scientists of KIT’s Institute of Nanotechnology (INT) and Karlsruhe Nano Micro Facility (KNMF), of the Helmholtz Institute Ulm (HIU) established jointly by KIT and Ulm University, and of the Indian Institute of Technology in Madras have now demonstrated the suitability of HEO as conversion materials for reversible lithium storage. Conversion batteries based on electrochemical material conversion allow for an increase of the stored amount of energy, while battery weight is reduced. The scientists used HEO to produce conversion-based electrodes that survived more than 500 charging cycles without any significant degradation of capacity.
Post link
Acting like a muscle, nano-sized device lifts 165 times its own weight
Materials scientists discover powerful effect that could benefit robotics, aviation, medicine and other fields
Imagine repeatedly lifting 165 times your weight without breaking a sweat – a feat normally reserved for heroes like Spider-Man.
New Brunswick engineers have discovered a simple, economical way to make a nano-sized device that can match the friendly neighborhood Avenger, on a much smaller scale. Their creation weighs 1.6 milligrams (about as much as five poppy seeds) and can lift 265 milligrams (the weight of about 825 poppy seeds) hundreds of times in a row.
Its strength comes from a process of inserting and removing ions between very thin sheets of molybdenum disulfide (MoS2), an inorganic crystalline mineral compound. It’s a new type of actuator – devices that work like muscles and convert electrical energy to mechanical energy.
The Rutgers discovery – elegantly called an “inverted-series-connected (ISC) biomorph actuation device” – is described in a study published online today in the journal Nature.
“We found that by applying a small amount of voltage, the device can lift something that’s far heavier than itself,” said Manish Chhowalla, professor and associate chair of the Department of Materials Science and Engineering in the School of Engineering. “This is an important finding in the field of electrochemical actuators. The simple restacking of atomically thin sheets of metallic MoS2 leads to actuators that can withstand stresses and strains comparable to or greater than other actuator materials.”
Post link
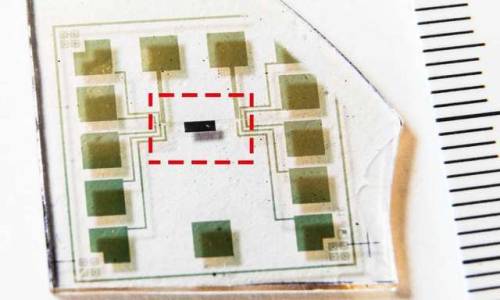
A major step forward in organic electronics
Researchers at the Laboratory of Organic Electronics, Linköping University, have developed the world’s first complementary electrochemical logic circuits that can function stably for long periods in water. This is a highly significant breakthrough in the development of bioelectronics.
The first printable organic electrochemical transistors were presented by researchers at LiU as early as 2002, and research since then has progressed rapidly. Several organic electronic components, such as light-emitting diodes and electrochromic displays, are already commercially available.
The most commonly used material is PEDOT:PSS, which is a p-type material in which the charge carriers are holes. In order to construct effective electron components, a complementary material, n-type, is required, in which the charge carriers are electrons. It has been difficult to find a sufficiently stable polymer material that can operate in water media and in which the long polymer chains can sustain high current when the material is doped.
In an article in Advanced Materials, Simone Fabiano and colleagues have presented results from an n-type conducting material in which the ladder-type structure of the polymer backbone favours ambient stability and high current when doped. One example is BBL, poly(benzimidazobenzophenanthroline), a material often used in solar cell research.
Post link