#gene expression
(Image caption: Lines labeling cortical subplate, mesencephalic, and diencephalic cell types (see Fig. 7 in Shima et al.))
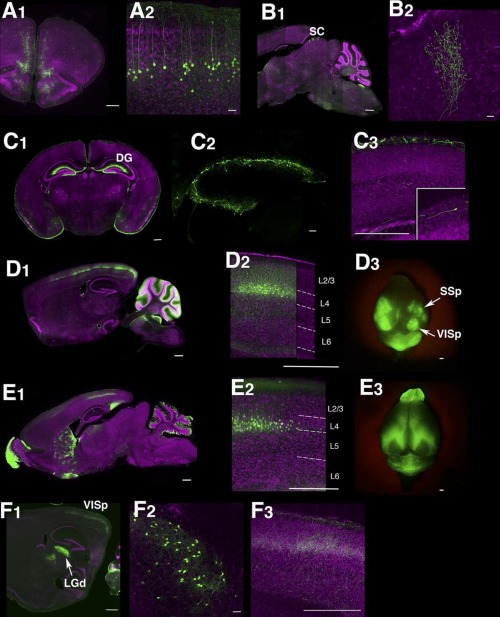
Trapping individual cell types in the mouse brain
The complexity of the human brain depends upon the many thousands of individual types of nerve cells it contains. Even the much simpler mouse brain probably contains 10,000 or more different neuronal cell types. Brandeis scientists Yasu Shima, Sacha Nelson and colleagues report in the journal eLife on a new approach for genetically identifying and manipulating these cell types.
Cells in the brain have different functions and therefore express different genes. Important instructions for which genes to express, in which cell types, lie not only in the genes themselves, but in small pieces of DNA called enhancers found in the large spaces between genes. The Brandeis group has found a way to highjack these instructions to express other artificial genes in particular cell types in the mouse brain. Some of these artificially expressed genes (also called transgenes) simply make the cells fluorescent so they can be seen under the microscope. Other transgenes are master regulators that can be used to turn on or off any other gene of interest. This will allow scientists to activate or deactivate the cells to see how they alter behavior, or to study the function of specific genes by altering them only in some cell types without altering them everywhere in the body. In addition to developing the approach, the Brandeis group created a resource of over 150 strains of mice in which different brain cell types can be studied.
Post link
Study reveals how differences in male and female brains emerge
Nematode worms may not be from Mars or Venus, but they do have sex-specific circuits in their brains that cause the males and females to act differently. According to new research published in Nature, scientists have determined how these sexually dimorphic (occurring in either males or females) connections arise in the worm nervous system. The research was funded by the NIH’s National Institute of Neurological Disorders and Stroke (NINDS).
“For decades, there has been little focus on the impact of sex on many areas of biomedical research,” said Coryse St. Hillaire-Clarke, Ph.D., program officer on this NINDS project. “This study helps us understand how sex can influence brain connectivity.”
In nematode worms, (known as Caenorhabditis elegans or C. elegans), a small number of neurons are found exclusively in male or female brains. The remaining neurons are found in both sexes, although their connection patterns are different in male and female brains. Oliver Hobert, Ph.D., professor of biological sciences at Columbia University in New York City, and his colleagues looked at how these wiring patterns form.
Dr. Hobert’s team observed that in the worms’ juvenile state, before they reach sexual maturity, their brain connections were in a hybrid, or mixed state, comprised of both male and female arrangements. As they reached sexual maturity, however, their brains underwent a pruning process, which got rid of particular connections and led to either male or female patterns.
“We found that differences in male and female brains develop from a ground state, which contains features of both sexes. From this developmental state, distinctly male or female features eventually emerge,” said Dr. Hobert.
Next, Dr. Hobert’s team showed that sex-specific wiring in the brain results in dimorphic behavior. They discovered that PHB neurons, chemosensory brain cells that detect chemical cues in the environment such as food, predators or potential mates, work differently in males and females. In males, these neurons proved to be important in recognizing mating cues while in females, the neurons helped them avoid specific taste cues. However, early in development, PHB neurons in males also responded to signals regulating taste, suggesting that even though those neurons are found in all nematodes, in adults, their functions differ as a result of sex-specific wiring in the brain.
Dr. Hobert’s team used genetically engineered nematodes to look more carefully at individual connections between brain cells. The researchers found that swapping the sex of individual neurons changed wiring patterns and influenced behavioral differences in males and females.
Additional experiments helped to identify genes involved in regulating the pruning process during development. Dr. Hobert’s group discovered that certain transcription factors, which are molecules that help control gene activity, are present in a dimorphic state and may help establish male or female connections in the brain. In future experiments, Dr. Hobert and his colleagues plan to examine how these molecules target specific connections for pruning.
Post link

Neuroscientists roll out first comprehensive atlas of brain cells
When you clicked to read this story, a band of cells across the top of your brain sent signals down your spine and out to your hand to tell the muscles in your index finger to press down with just the right amount of pressure to activate your mouse or track pad.
A slew of new studies now shows that the area of the brain responsible for initiating this action — the primary motor cortex, which controls movement — has as many as 116 different types of cells that work together to make this happen.
The 17 studies, appearing online in the journal Nature, are the result of five years of work by a huge consortium of researchers supported by the National Institutes of Health’s Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative to identify the myriad of different cell types in one portion of the brain. It is the first step in a long-term project to generate an atlas of the entire brain to help understand how the neural networks in our head control our body and mind and how they are disrupted in cases of mental and physical problems.
“If you think of the brain as an extremely complex machine, how could we understand it without first breaking it down and knowing the parts?” asked cellular neuroscientist Helen Bateup, a University of California, Berkeley, associate professor of molecular and cell biology and co-author of the flagship paper that synthesizes the results of the other papers. “The first page of any manual of how the brain works should read: Here are all the cellular components, this is how many of them there are, here is where they are located and who they connect to.”
Individual researchers have previously identified dozens of cell types based on their shape, size, electrical properties and which genes are expressed in them. The new studies identify about five times more cell types, though many are subtypes of well-known cell types. For example, cells that release specific neurotransmitters, like gamma-aminobutyric acid (GABA) or glutamate, each have more than a dozen subtypes distinguishable from one another by their gene expression and electrical firing patterns.
While the current papers address only the motor cortex, the BRAIN Initiative Cell Census Network (BICCN) — created in 2017 — endeavors to map all the different cell types throughout the brain, which consists of more than 160 billion individual cells, both neurons and support cells called glia. The BRAIN Initiative was launched in 2013 by then-President Barack Obama.
“Once we have all those parts defined, we can then go up a level and start to understand how those parts work together, how they form a functional circuit, how that ultimately gives rise to perceptions and behavior and much more complex things,” Bateup said.
Knock-in mice
Together with former UC Berkeley professor John Ngai, Bateup and UC Berkeley colleague Dick Hockemeyer have already used CRISPR-Cas9 to create mice in which a specific cell type is labeled with a fluorescent marker, allowing them to track the connections these cells make throughout the brain. For the flagship journal paper, the Berkeley team created two strains of “knock-in” reporter mice that provided novel tools for illuminating the connections of the newly identified cell types, she said.
“One of our many limitations in developing effective therapies for human brain disorders is that we just don’t know enough about which cells and connections are being affected by a particular disease and therefore can’t pinpoint with precision what and where we need to target,” said Ngai, who led UC Berkeley’s Brain Initiative efforts before being tapped last year to direct the entire national initiative. “Detailed information about the types of cells that make up the brain and their properties will ultimately enable the development of new therapies for neurologic and neuropsychiatric diseases.”
Ngai is one of 13 corresponding authors of the flagship paper, which has more than 250 co-authors in all.
Bateup, Hockemeyer and Ngai collaborated on an earlier study to profile all the active genes in single dopamine-producing cells in the mouse’s midbrain, which has structures similar to human brains. This same profiling technique, which involves identifying all the specific messenger RNA molecules and their levels in each cell, was employed by other BICCN researchers to profile cells in the motor cortex. This type of analysis, using a technique called single-cell RNA sequencing, or scRNA-seq, is referred to as transcriptomics.
The scRNA-seq technique was one of nearly a dozen separate experimental methods used by the BICCN team to characterize the different cell types in three different mammals: mice, marmosets and humans. Four of these involved different ways of identifying gene expression levels and determining the genome’s chromatin architecture and DNA methylation status, which is called the epigenome. Other techniques included classical electrophysiological patch clamp recordings to distinguish cells by how they fire action potentials, categorizing cells by shape, determining their connectivity, and looking at where the cells are spatially located within the brain. Several of these used machine learning or artificial intelligence to distinguish cell types.
“This was the most comprehensive description of these cell types, and with high resolution and different methodologies,” Hockemeyer said. “The conclusion of the paper is that there’s remarkable overlap and consistency in determining cell types with these different methods.”
A team of statisticians combined data from all these experimental methods to determine how best to classify or cluster cells into different types and, presumably, different functions based on the observed differences in expression and epigenetic profiles among these cells. While there are many statistical algorithms for analyzing such data and identifying clusters, the challenge was to determine which clusters were truly different from one another — truly different cell types — said Sandrine Dudoit, a UC Berkeley professor and chair of the Department of Statistics. She and biostatistician Elizabeth Purdom, UC Berkeley associate professor of statistics, were key members of the statistical team and co-authors of the flagship paper.
“The idea is not to create yet another new clustering method, but to find ways of leveraging the strengths of different methods and combining methods and to assess the stability of the results, the reproducibility of the clusters you get,” Dudoit said. “That’s really a key message about all these studies that look for novel cell types or novel categories of cells: No matter what algorithm you try, you’ll get clusters, so it is key to really have confidence in your results.”
Bateup noted that the number of individual cell types identified in the new study depended on the technique used and ranged from dozens to 116. One finding, for example, was that humans have about twice as many different types of inhibitory neurons as excitatory neurons in this region of the brain, while mice have five times as many.
“Before, we had something like 10 or 20 different cell types that had been defined, but we had no idea if the cells we were defining by their patterns of gene expression were the same ones as those defined based on their electrophysiological properties, or the same as the neuron types defined by their morphology,” Bateup said.
“The big advance by the BICCN is that we combined many different ways of defining a cell type and integrated them to come up with a consensus taxonomy that’s not just based on gene expression or on physiology or morphology, but takes all of those properties into account,” Hockemeyer said. “So, now we can say this particular cell type expresses these genes, has this morphology, has these physiological properties, and is located in this particular region of the cortex. So, you have a much deeper, granular understanding of what that cell type is and its basic properties.”
Dudoit cautioned that future studies could show that the number of cell types identified in the motor cortex is an overestimate, but the current studies are a good start in assembling a cell atlas of the whole brain.
“Even among biologists, there are vastly different opinions as to how much resolution you should have for these systems, whether there is this very, very fine clustering structure or whether you really have higher level cell types that are more stable,” she said. “Nevertheless, these results show the power of collaboration and pulling together efforts across different groups. We’re starting with a biological question, but a biologist alone could not have solved that problem. To address a big challenging problem like that, you want a team of experts in a bunch of different disciplines that are able to communicate well and work well with each other.”
(Image caption: Brain slice from a transgenic mouse, in which genetically defined neurons in the cerebral cortex are labeled with a red fluorescent reporter gene. Credit: Tanya Daigle, courtesy of the Allen Institute)

Mid-pregnancy may be defining period for human brain
About four or five months after conception, a burst of synaptic growth begins in the prefrontal cortex (PFC) of the human fetus. And within this tangled mass of connections, the developing brain acquires the unique properties that make humans capable of abstract thought, language, and complex social interactions.
But what are the molecular ingredients needed for this flowering of synapses to occur and that lead to such profound changes in the brain? In two new papers published in the journal Nature, Yale researchers have identified key changes in gene expression and structure within the developing human brain that make it unique among all animal species.
These insights could have profound implications for understanding common developmental or brain disorders, researchers say.
“It is surprising and somewhat disappointing that we still don’t know what makes the human brain different from the brains of other closely related species,” said Nenad Sestan, the Harvey and Kate Cushing Professor of Neuroscience at Yale, professor of comparative medicine, of genetics and of psychiatry, and senior author of both papers. “Knowing this is not just an intellectual curiosity to explain who we are as a species — It may also help us better understand neuropsychiatric disorders such as schizophrenia and autism.”
For the studies, Sestan’s lab team conducted an extensive analysis of the gene expressions that occur in the prefrontal cortexes of humans, macaque monkeys, and mice midway through fetal development, identifying both similarities and differences between the species.
A critical factor in determining outcomes in the developing brain for all of these species, they found, is the concentration of retinoic acid, or RA, a byproduct of Vitamin A. Retinoic acid, which is essential for the development of every organ, is tightly regulated in all animals. Too much or too little RA can lead to developmental abnormalities.
In the first paper, a research team led by Mikihito Shibata and Kartik Pattabiraman, both from Yale School of Medicine, found that RA is increased in the prefrontal cortex of humans, mice, and macaques alike during the second trimester, the most crucial time for formation of neural circuitry and connections.
When researchers blocked RA signals in the prefrontal cortex of mice, the animals failed to develop the specific circuits and connectivity in areas of the brain that in humans are essential for working memory and cognition. In humans, this same pathway is also disrupted during development in patients with schizophrenia and autism spectrum disorders, suggesting these disorders may share similar roots during development.
However, a close examination of the gene CYP26B1, which both synthesizes and turns off RA in the prefrontal cortex, revealed important differences between mice and primates. For instance, in mice the gene limits activity of RA beyond the animal’s tiny prefrontal cortex. When researchers blocked this gene in the mice, areas of their brains associated with sensory and motor skills came to resemble the synaptic wiring found in the prefrontal cortex. This finding further affirms the crucial role played by RA in the expansion of the prefrontal cortex — and in promoting ever greater brain complexity — in humans and other primates.
“RA is the first domino to fall, which sets in motion the complex gene networks which lead to development of brain areas associated with human thought,” said Pattabiraman, a clinical fellow in the Yale Child Study Center and co-author of both papers.
Researchers then asked how retinoic acid works this magic.
The development of the human brain is marked by the burst of synaptic growth during the second trimester. These connections start in the PFC but gradually diminish as they approach sensory and motor neurons towards the rear of the brain.
To better understand why that is, Shibata and Pattabiraman for the second study focused on the gene CBLN2, which is enriched in the prefrontal cortex and plays a key role in forming these connections. The gene is also directly regulated by RA. They found that CBLN2 is switched on earlier in the front of the developing human brain than in other parts of the brain. Furthermore, they found that the gene is expressed longer and over a wider area of the human brain than in macaques or mice, suggesting a central role of the PFC in the emergence of human-specific properties.
The researchers also identified a small genomic deletion near the CBLN2 gene which has been conserved in the evolution of human and chimpanzees but not in other animals. To see whether these deletions played a role in growth of PFC connections, they introduced the deletions into the mouse genome. Mice possessing these deletions showed a human-like expansion of CBLN2 and a 30% increase of connections in the adult mouse PFC.
Taken together, the two papers show that the path to understanding the genetic mechanisms underlying advanced cognitive ability starts with the localized production of RA, which then activates various downstream genes, including CBLN2. This dictates where and when these crucial brain connections are formed.
“The prefrontal cortex integrates the information from other parts of the central nervous system and provides top-down control of attention, thought, emotions and actions,” Sestan said. “It is also central to dysfunctions in many neuropsychiatric disorders. The subtle changes in the connections that create the human mind may make it sick as well.”
(Image caption: The green areas represent the prefrontal cortex of a mouse brain. Yale researchers investigated why it is so greatly expanded in humans and mice)
Biochemistry & Molecular Biology, University of Oklahoma Health Sciences Center
A computational system for automated meta-analysis of gene expression data.
The Gland that has got a SecreteSecret
This article will focus on one of the more important glads of the human body; the thyroid. This article will focus on the anatomy and physiology, biochemistry and clinical aspects of the thyroid, hopefully giving our readers a better understanding of this organ.
The Thyroid
Situated on the ventral side of the neck, the thyroid gland is composed of two lobes: right and left that are situated anterolaterally to the trachea. It normally weighs 15 to 20 grams in adults (1), but despite its small size, it is responsible for producing two important bodily hormones.
Follicular cells in the thyroid gland mainly produce the prohormone thyroxine (T4), and a smaller amount of the active hormone, triiodothyronine (T3). Most T4 is converted to T3 in other tissues by thyroxine-specific deiodinase enzymes, activating it when it reaches its target site.
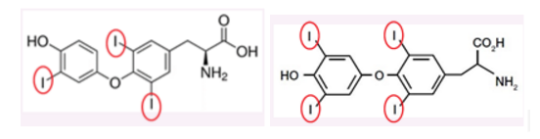
Figure 1. Showing the molecular structure of T3 (left) and T4 (right).
T3 and T4 from thyroid gland to target tissue
Synthesised T3 and T4 diffuse out of follicular cells and enter a blood vessel. Almost all secreted T3 and T4 circulating the bloodstream are bound to proteins; the major binding protein being thyroxine-binding globulin (TBG). A TBG-blood test(2) may be used to diagnose problems with the thyroid such as hypothyroidism, a clinical condition where insufficient production of thyroid hormone occurs.
Free T3 and T4 enter cells by active transport, an energy-dependent transport method. As discussed above, organ tissues with high blood flow (such as liver, skeletal muscles and kidney) possess enzyme deiodinase and catalyses most of the conversion of T3 and T4. Other tissues with low local T3 generation may depend on these tissues to obtain sufficient levels of T3.
At the physiological level
The most important role of thyroid hormones are to control basal metabolic rate (BMR). BMR refers to the basal rate of oxygen consumption and heat production. Normally, mitochondria generate energy by oxidative phosphorylation. During this process, the energy from protons (H+) moving down a proton gradient is used to generate ATP (the energy currency of the cell). This is a similar process to the momentum of water being harnessed by water wheels in old mills.However, a special type of protein, called the uncoupling protein (UCP), is found exclusively in brown adipose tissue (BAT). Mitochondria in these cells can provide an alternative pathway for protons to travel back inside the mitochondria, down their proton gradient. This alternative pathway results in no ATP production with the energy being dissipated as heat.(4)
In the cardiovascular system, thyroid hormones increase the gene expression for β1-adrenergic receptors in cardiac muscle cells and increase the responsiveness of these cells towards β adrenergic activity. The overall effect increases the force of myocardium contraction (positive inotropy) and rate of heart muscle contraction (positive chronotropy), increasing cardiac output and blood vessel dilation in the skin, muscle and heart. The hormone increases tissue sensitivity to beta adrenergic hormones, increasing the heart rate and force of contraction.
Thyroxine hormone also affects the other systems such as the respiratory system, skeletal system, reproduction and nervous system. However, the most important functions of thyroid hormone are the regulation of BMR, maturation and development of nervous system and increase responsiveness of tissue to adrenergic activity.
Mechanism of thyroid hormone
The steps below correspond to the numbers in Figure 2.
1) T3 diffuses into the cytosol and subsequently into the nucleus (8).
2)Thyroid hormone receptor (TR) is located in the nucleus prebound to DNA. TR usually dimerises with a retinoid X receptor (RXR) and this dimer recognises and binds at a specific site on DNA known as the Thyroid Response Element (TRE). TH binds to TR leading to the dissociation of co-repressors (Figure 2).
3)At the same time, recruitment of co-activators (Figure 2) occurs.
4)The TRE mentioned in step 2 is a segment of DNA known as the refulatory sequence, a segment of DNA that increases or decreases the expression of specific genes. In this case, when the T3 binds to the TR-RXR dimer, and the TRE may activate or repress the target genes.
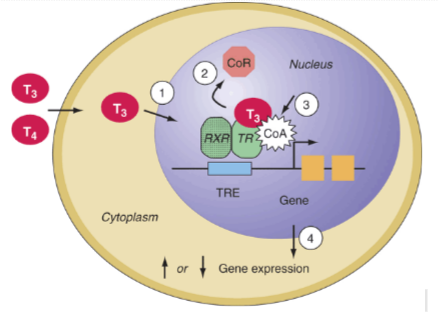
Figure 2. Diagram shows a schematic diagram of the general biochemical action of thyroid hormones on the target DNA
Transcription is followed by RNA translation to form hundreds of new intracellular proteins. T3 changes the rate of expression for hundreds of genes and increases or decreases the production of structural and functional proteins which may be the key molecules in different metabolic processes. T4 also performs such function, but is less potent than its counterpart T3.
With such function, one can imagine just how important the level of thyroid hormone is in the regulation of different physiological processes and how this may impact upon health (9)
Regulation Of Thyroid Hormone Production And Secretion
A hormone with such varied functionality has to be regulated to ensure its adequately supplied to targeted organs. Such intricate control has to be performed by the “endocrine master”; the hypothalamus. The hypothalamus releases thyrotropin hormone (TRH), which stimulates the release of thyroid stimulating hormone (TSH) in the closely linked anterior lobe of pituitary gland. TSH is then transported in the blood where it binds to the TSH receptor on the thyroid gland. TSH speeds up the production and release of thyroid hormones, promoting the growth of the gland with the help of some other growth factors.
When thyroid hormone levels are in excess, circulating molecules act on the hypothalamus and pituitary gland to decrease TRH and TSH secretion respectively. The mechanism involved is a negative-feedback control mechanism. When TRH and TSH secretion decrease, so does the production and the secretion of thyroid hormones. The hormones drop until the optimal physiological level whereby the inhibitions on TRH and TSH secretion are lifted (Figure 3).
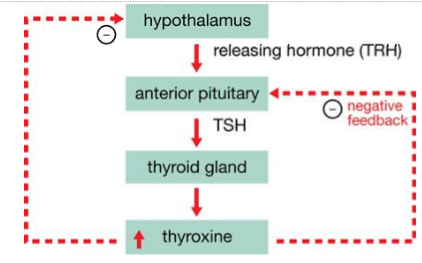
Figure 3. Image shows the regulation of thyroid hormone by the hypothalamus and pituitary gland in a negative-feedback loop.
Diseases Related To Thyroid Gland
T3 (Figure 1) contains three iodine atoms. The synthesis of thyroid hormones requires an adequate supply of dietary iodine. The recommended dietary allowance (RDA) for iodine in an adult male is 150µg and slightly higher in pregnant women, 220µg (5). Deficiency of this precursor leads to insufficient production of T3 and T4. The consequences of this are low levels of circulating thyroid hormones which cause an increase of TSH secretion from the pituitary gland interfering with the negative feedback. Increased stimulation of TSH increases the activity of the thyroid gland in an attempt to normalize thyroid hormone level (6). Consequently the gland grows larger than the normal size, producing a condition known as a goitre(Figure 4).
A goitre refers to the enlargement of the thyroid gland (Figure 4), this could be due to hypothyroidism or hyperthyroidism. Goitres are more common in population living in mountainous regions, where access to iodine sources such as seafood are restricted. Such dietary deficiency can be prevented by adding small amounts of iodine to table salt.
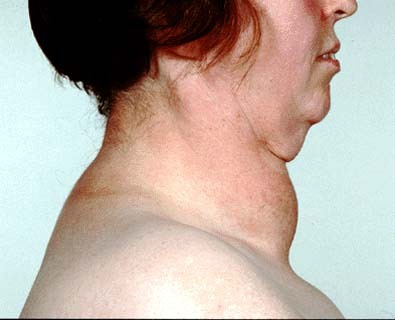
Figure 4. Image showing a patient presenting a goitre.
Conclusion
Hopefully after reading this article, you’re a little more in the know about the little gland secretlysecreting hormones to help you stay healthy. Next time you’re calorie counting or checking the nutritional content of your food, make sure that you’re getting enough iodine in your diet as it can ensure that you don’t end up with a large number of problems down the line.
Post link