#phase transitions
Solid + solid = liquid?
Can a mix of solids form a liquid? In a “deep eutectic system” (DES), yes. In these cases—which includes some mixtures of sugars, amino acids, and organic acids—compounds that are typically solid at room temperature lower each other’s melting points when combined in some ratios. As a result, they melt. Starting at the left, this picture shows two natural compounds, menthol and lauric acid. Combined in a certain molar fraction as they were heated and stirred, the compounds formed the eutectic liquid in the flask on the right. After the liquid is formed, it remains stable at room temperature. This is also what happens with honey, a naturally occurring DES that is a viscous liquid resulting from the mixture of various sugars. Project Des.Solve, funded by the European Research Council, aims to extend knowledge of these systems, focusing on their characterization and boosting application to fields including extraction, biocatalysis, green chemistry, and biomedical science. — Craig Bettenhausen
Submitted by Liane Meneses and Luísa Pereira/Project Des.Solve
Do science. Take pictures. Win money. Enter our photo contest here.
Post link
Ultrafast optical fiber-based electron gun to reveal atomic motions
One of the most enduring “Holy Grail” experiments in science has been attempts to directly observe atomic motions during structural changes. This prospect underpins the entire field of chemistry because a chemical process occurs during a transition state—the point of no return separating the reactant configuration from the product configuration.
What does that transition state look like and, given the enormous number of different possible nuclear configurations, how does a system even find a way to make it happen?
Now in the journal Applied Physics Letters, researchers at the Max Planck Institute for the Structure and Dynamics of Matter are reporting “ultrabright” electron sources with sufficient brightness to literally light up atomic motions in real time—at a time scale of 100 femtoseconds, making these sources particularly relevant to chemistry because atomic motions occur in that window of time.
After seeing the first atomic movies of phase transitions in bulk thin films using high-energy (100 kilovolt) electron bunches, the researchers wondered if they could achieve atomic resolution of surface reactions—occurring within the first few monolayers of materials—to gain a better understanding of surface catalysis.
Post link
Researcher pushes limit of when water will freeze
Though it is one of the great mysteries of science, the transformation of water into ice often escapes people’s minds as it is just assumed that’s what happens. But how and why it happens is the subject of intense scrutiny by ice scientists like Hadi Ghasemi, Cullen Associate Professor of Mechanical Engineering at the University of Houston. In order to watch the process of crystallization of water into ice at the molecular level, Ghasemi is reporting the best look yet at the process: water-ice phase transformation down to 2 nm (nanometers) in diameter.
Then when Ghasemi examined these tiny particles, he made another discovery. He could break the limit of when water freezes and maintain the tiny droplets as liquid by putting them in contact with soft interfaces, like gels or lipids.
“We found that if a water droplet is in contact with a soft interface, freezing temperature could be significantly lower than hard surfaces. Also, a few-nanometer water droplet could avoid freezing down to -44 C if it is in contact with a soft interface,” Ghasemi reports in Nature Communications.
Post link
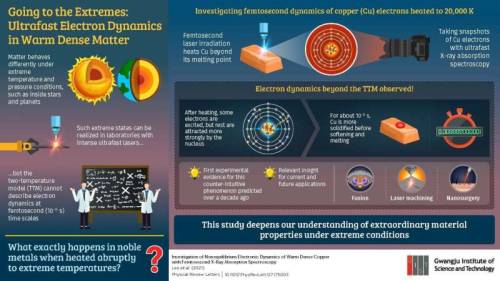
Scientists reveal ultrafast melting dynamics in matter heated to extreme temperatures
Ordinary matter behaves very differently when subjected to extreme temperatures and pressures, such as that inside stellar and planetary cores. Conventional rules of condensed matter physics and plasma physics are not applicable in such scenarios. In particular, an extreme state known as “warm dense matter” (WDM) straddles the boundary of condensed matter physics and plasma physics.
One might think that such states can never be created in a terrestrial setting. But, in fact, short laser pulses that are only femtoseconds (10-15 s, or a quadrillionth of a second) long are intense enough to recreate such conditions in a laboratory. Conventional physical models that describe such states typically assume that electrons become excited by the laser pulse attain equilibrium within tens of femtoseconds while the ions remain “cold.” However, in doing so, the non-equilibrium dynamics of the electrons are completely disregarded.
Post link
Aberrant electronic and structural alterations in pressure-tuned perovskite
The perovskite NaOsO3 has a complicated but interesting temperature-dependent metal-insulator transition (MIT). A team led by Drs. Raimundas Sereika and Yang Ding from the Center for High Pressure Science and Technology Advanced Research (HPSTAR) showed that the insulating ground state in NaOsO3 can be preserved up to at least 35 GPa with a sluggish MIT reduction from 410 K to a near room temperature and possible transformation to a polar phase. The work has been published in npj Quantum Materials.
NaOsO3 perovskite undergoes a metal-insulator transition concomitant with the onset of an antiferromagnetic long-range ordering at a Neel temperature of about 410 K, which is accompanied by a magnetic ordering without any lattice distortion.
The team carried out a combined experimental and computational study to understand the effect of external pressure on perovskite NaOsO3. They found hidden hysteretic resistance properties with a transient metallic state near 200 K. Also three electronic character anomalies (at 1.7, 9.0, and 25.5 GPa), and a structural transition to the singular polar phase (at ~ 18 GPa) were discovered.
Post link
Liquid carbon characterized using a free electron laser
From common soot to precious diamonds, carbon is familiar in many guises, but there have been little more than glimpses of carbon in the liquid form. Researchers at the FERMI Free Electron Laser (FEL) source have now not only generated a liquid carbon sample, but have characterized its structure, tracking the ultrafast rearrangements of electron bonding and atomic coordinates that take place as their carbon samples melt. “As far as I know, that is the fastest structural transition in condensed matter,” says Emiliano Principi, principal investigator on the project.
The work fills in some of the gaps in the element’s phase diagram—a plot of its phases at different temperatures and pressures. Despite the ubiquity of carbon and the interest it garners in so many facets of science—from sensorsandsolar cellstoquantum computingandspace rocket protection systems—knowledge of its phase diagram remains patchy. Typically, as soon as solid carbon can’t take the heat, it sublimates to gas. For other materials, researchers can enroll high-pressure cells to prevent the sample expanding straight into a gas at high temperatures, but these are usually diamond, precisely the element the conditions are designed to melt.
Post link
Controlling heat flow in a solid by switching crystal structure dimensionality
Just as an electrical switch regulates the flow of electric current, thermal switches can control the flow of heat. These switches serve as thermal control devices and are useful for thermal management applications. For example, they can be used in industries to reduce waste heat, resulting in cost and energy savings. These switches require materials whose thermal conductivity (κ) can be modulated to a large extent. This would allow the switch to have an “on” and “off” state depending on the thermal conductivity. However, such materials are rare and challenging to develop, and those that have been developed show only small reversible variations in their κ.
Now, in a study published in Advanced Electronic Materials, researchers from Tokyo Institute of Technology (Tokyo Tech) and the National Institute for Materials Science, Japan, have taken things to the next level with a material that can achieve a large variation in its κ by changing its crystal structure dimensionality. The team achieved this remarkable feat by using a solid solution of lead selenide (PbSe) and tin selenide (SnSe), which can switch between a 3-dimensional (3D) cubic crystal structure and a 2-dimensional (2D) layered crystal structure with changes in temperature.
Post link