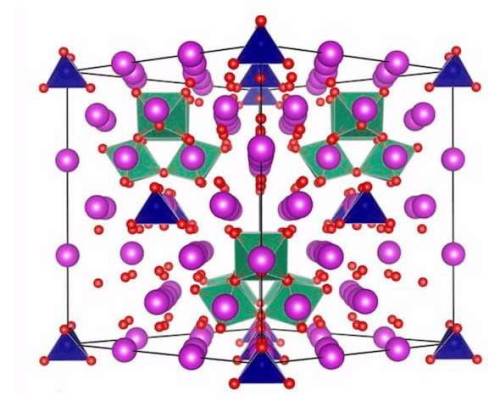
#crystal structure
Fine-tuning chemistry by doping with transition metals produced stability in bismuth oxide
ANSTO has contributed to research led by the University of Sydney, involving doping transition metals in a polymorph of bismuth oxide in a search for more structural stability.
Cubic high-temperature polymorph of bismuth oxide, δ-Bi2O3, is the best known oxide ionic conductor but its narrow stability range (729—817 °C), which is close to its melting temperature of 817 °C precludes its practical use.
A large collaboration, led by Professor Chris Ling and Dr. Julia Wind (as part of her Ph.D.) from the University of Sydney involving researchers from ANSTO and two other universities, has achieved the design and understanding of the complex crystal structure and chemistry behind a commensurate structure within the fast-ion conducting stabilised bismuth oxide, co-doped with chromium and niobium, Bi23CrNb3O45.
The study was published in the Chemistry of Materials.
Post link
Superstrong Al alloys may change manufacturing processes for automobiles, aerospace devices
Purdue University researchers have developed a superstrong material that may change some manufacturing processes for the aerospace and automobile industries.
The Purdue team, led by Xinghang Zhang, a professor in Purdue’s School of Materials Engineering, created high-strength aluminum alloy coatings. According to Zhang, there is an increasing demand for such materials because of their advantages for automakers and aerospace industries.
“We have created a very durable and lightweight aluminum alloy that is just as strong as, and possibly stronger than, stainless steel,” Zhang said. “Our aluminum alloy is lightweight and provides flexibility that stainless steel does not in many applications.”
Another member of the Purdue team, Yifan Zhang, a graduate student in materials engineering, said the aluminum alloy they created could be used for making wear- and corrosion-resistant automobile parts such as engines and coatings for optical lenses for specialized telescopes in the aerospace industry.
Post link
Copper ions flow like liquid through crystalline structures
Materials scientists have sussed out the physical phenomenon underlying the promising electrical properties of a class of materials called superionic crystals. A better understanding of such materials could lead to safer and more efficient rechargeable batteries than the current standard-bearer of lithium ion.
Becoming a popular topic of study only within the past five years, superionic crystals are a cross between a liquid and a solid. While some of their molecular components retain a rigid crystalline structure, others become liquid-like above a certain temperature, and are able to flow through the solid scaffold.
In a new study, scientists from Duke University, Oak Ridge National Laboratory (ORNL) and Argonne National Laboratory (ANL) probed one such superionic crystal containing copper, chromium and selenium (CuCrSe2) with neutrons and X-rays to determine how the material’s copper ions achieve their liquid-like properties. The results appear online on Oct. 8 in the journal Nature Physics.
“When CuCrSe2 is heated above 190 degrees Fahrenheit, its copper ions fly around inside the layers of chromium and selenium about as fast as liquid water molecules move,” said Olivier Delaire, associate professor of mechanical engineering and materials science at Duke and senior author on the study. “And yet, it’s still a solid that you could hold in your hand. We wanted to understand the molecular physics behind this phenomenon.”
Post link
Engineers develop new software tool to aid material modeling research
A new software tool can accelerate materials science research by cutting out tedious background research on material properties. Penn State and Sandia National Laboratories researchers recently debuted propSym, an open-source software on the programming platform MATLAB, to calculate the fundamental constants needed to describe the physical properties of solids, such as metals, ceramics or composites.
Researchers input a material’s physical characteristics and structure, and the program produces its fundamental property constants—key values researchers need to model various materials.
“Some physical models contain hundreds or thousands of redundant components, which can make the model overwhelming,” said Anubhav Roy, a doctoral student in engineering science and mechanics in the Penn State College of Engineering and first author on the paper. “The program is able to greatly reduce the number of components for any physical property that is connected to solids with inherent crystalline symmetry.”
Post link
Researchers discover a novel layered superconductor based on tin and arsenic
The layered superconducting material is characterized by a crystal structure in which a SnAs layer (wherein Sn and As are two-dimensionally bonded to develop superconductivity) and a Na layer (the spacer layer) are alternately laminated. Considering that such a layered structure is similar to that of a cuprate- or iron-based high-temperature (high-Tc) superconductor, it is possible that in SnAs-based layered materials, superconductivity is developed as a result of the unconventional pairing mechanism.
The research group of Yoshikazu MIZUGUCHI (Associate Professor) and Yosuke GOTO (Project Researcher) specializes in the discovery of two-dimensional layered structures—various materials can be designed by laminating different kinds of layers. As an example, in 2012, this research group also reported the discovery of novel layered superconducting material systems, Bi4O4S3 and LaO1-xFxBiS2, based on bismuth (Bi) and sulfur (S). To add, as the two-dimensional crystal structure gives rise to a low-dimensional electronic state, researchers are actively studying unique quantum phenomena such as high-temperature superconductivity.
Regarding NaSn2As2, Mizuguchi asserts that although the transition temperature of 1.3 K (-271.85°C) is not exactly high, it is anticipated that new materials will be developed based on the SnAs conductive layer, thereby clarifying the mechanisms underlying the increase of the transition temperature as well as those responsible for high-transition-temperature superconductivity.
Post link
Chemistry Key to Future Jet Engines
The Periodic Table may not sound like a list of ingredients but, for a group of materials scientists, it’s the starting point for designing the perfect chemical make-up of tomorrow’s jet engines.
Inside a jet engine is one of the most extreme environments known to engineering.
In less than a second, a ton of air is sucked into the engine, squeezed to a fraction of its normal volume and then passed across hundreds of blades rotating at speeds of up to 10,000 rpm; reaching the combustor, the air is mixed with kerosene and ignited; the resulting gases are about a third as hot as the sun’s surface and hurtle at speeds of almost 1,500 km per hour towards a wall of turbines, where each blade generates power equivalent to the thrust of a Formula One racing car.
Read more: http://www.laboratoryequipment.com/videos/2015/06/chemistry-key-future-jet-engines
Helium ‘balloons’ offer new path to control complex materials
Researchers at the Department of Energy’s Oak Ridge National Laboratory have developed a new method to manipulate a wide range of materials and their behavior using only a handful of helium ions.
The team’s technique, published in Physical Review Letters, advances the understanding and use of complex oxide materials that boast unusual properties such as superconductivity and colossal magnetoresistance but are notoriously difficult to control.
For the first time, ORNL researchers have discovered a simple way to control the elongation of a crystalline material along a single direction without changing the length along the other directions or damaging the crystalline structure. This is accomplished by adding a few helium ions into a complex oxide material and provides a never before possible level of control over magnetic and electronic properties.
“By putting a little helium into the material, we’re able to control strain along a single axis,” said ORNL’s Zac Ward, who led the team’s study. “This type of control wasn’t possible before, and it allows you to tune material properties with a finesse that we haven’t previously had access to.”
Post link
Study shows how calcium carbonate forms composites to make strong materials such as in shells and pearls
Seashells and lobster claws are hard to break, but chalk is soft enough to draw on sidewalks. Though all three are made of calcium carbonate crystals, the hard materials include clumps of soft biological matter that make them much stronger. A study today in Nature Communications reveals how soft clumps get into crystals and endow them with remarkable strength.
The results show that such clumps become incorporated via chemical interactions with atoms in the crystals, an unexpected mechanism based on previous understanding. By providing insight into the formation of natural minerals that are a composite of both soft and hard components, the work will help scientists develop new materials for a sustainable energy future, based on this principle.
“This work helps us to sort out how rather weak crystals can form composite materials with remarkable mechanical properties,” said materials scientist Jim De Yoreo of the Department of Energy’s Pacific Northwest National Laboratory. “It also provides us with ideas for trapping carbon dioxide in useful materials to deal with the excess greenhouse gases we’re putting in the atmosphere, or for incorporating light-responsive nanoparticles into highly ordered crystalline matrices for solar energy applications.”
Post link
In 1974, Roger Penrose, a British mathematician, created a revolutionary set of tiles that could be used to cover an infinite plane in a pattern that never repeats. In 1982, Daniel Shechtman, an Israeli crystallographer, discovered a metallic alloy whose atoms were organized unlike anything ever observed in materials science. Penrose garnered public renown on a scale rarely seen in mathematics. Shechtman won the Nobel Prize. Both scientists defied human intuition and changed our basic understanding of nature’s design, revealing how infinite variation could emerge within a highly ordered environment.
At the heart of their breakthroughs is “forbidden symmetry,” so-called because it flies in the face of a deeply ingrained association between symmetry and repetition. Symmetry is based on axes of reflection—whatever appears on one side of a line is duplicated on the other. In math, that relationship is reflected in tiling patterns. Symmetrical shapes such as rectangles and triangles can cover a plane with neither gap nor overlap, and in an ever-repeating pattern. Repeated patterns are called “periodic” and are said to have “translational symmetry.” If you move a pattern from place to place, it looks the same.
Penrose, a bold, ambitious scientist, was interested less in identical patterns and repetition, and more in infinite variation. To be precise, he was interested in “aperiodic” tiling, or sets of tiles that can cover an infinite plane with neither gap nor overlap, without the tiling pattern ever repeating itself. That was a challenge because he couldn’t use tiles with two, three, four, or six axes of symmetry—rectangles, triangles, squares, and hexagons—because on an infinite plane they would result in periodic or repeated patterns. That meant he had to rely on shapes believed to leave gaps in the tiling of a plane—those with forbidden symmetries.
Read more: http://nautil.us/issue/13/symmetry
Post link
Researchers gain a better understanding of the transformation of steel
Heating iron can alter its structure and is one of the methods for making various types of steel with different properties. That process is similar to the formation of frost flowers: one iron crystal structure transforms into another at a nucleus point, and the process expands further from that point. This type of nucleation in materials has the largest impact on their final properties, but is still the least understood in the field of metallurgy. For example, we still know little about how and where exactly this nucleation starts. Researchers at TU Delft have now shed new light on this subject in their publication ‘Preferential Nucleation during Polymorphic Transformations’ in Scientific Reports(Nature) of Wednesday 3 August.
The researchers demonstrated this nucleation process live by heating an iron sample to 1,000 degrees in a specially produced furnace at the European Synchrotron Radiation Facility in Grenoble, and monitoring the transformation process using X-rays. In this way, they were able to identify the places and their properties that were the most likely starting points for the nucleation process of the transition from ferrite to austenite.
Post link
Peering at the crystal structure of lithium
Elemental metals usually form simple, close-packed crystalline structures. Though lithium (Li) is considered a typical simple metal, its crystal structure at ambient pressure and low temperature remains unknown.
Lawrence Livermore National Laboratory (LLNL) researchers recently came up with a technique to obtain structural information for Li at conditions where traditional crystallographic methods are insufficient. Using this methodology, a decades-long puzzle finally may be solved.
Li is the lightest metal and least dense solid element at ambient conditions. Li and its compounds have several industrial applications, including heat-resistant glass and ceramics, lithium grease lubricants, flux additives for iron, steel and aluminum production, lithium batteries and lithium-ion batteries. These uses consume more than three quarters of lithium production.
“The superconductivity of alkali metals, and Li, is an issue that has been debated for many years,” said Stanimir Bonev, LLNL lead author of a paper appearing in a recent edition of Proceedings of the National Academy of Sciences. “Only recently superconductivity in Li at ambient pressure was observed. But to understand the superconducting properties, it is essential to know the crystal structure.”
Post link
Controlling heat flow in a solid by switching crystal structure dimensionality
Just as an electrical switch regulates the flow of electric current, thermal switches can control the flow of heat. These switches serve as thermal control devices and are useful for thermal management applications. For example, they can be used in industries to reduce waste heat, resulting in cost and energy savings. These switches require materials whose thermal conductivity (κ) can be modulated to a large extent. This would allow the switch to have an “on” and “off” state depending on the thermal conductivity. However, such materials are rare and challenging to develop, and those that have been developed show only small reversible variations in their κ.
Now, in a study published in Advanced Electronic Materials, researchers from Tokyo Institute of Technology (Tokyo Tech) and the National Institute for Materials Science, Japan, have taken things to the next level with a material that can achieve a large variation in its κ by changing its crystal structure dimensionality. The team achieved this remarkable feat by using a solid solution of lead selenide (PbSe) and tin selenide (SnSe), which can switch between a 3-dimensional (3D) cubic crystal structure and a 2-dimensional (2D) layered crystal structure with changes in temperature.
Post link
Team tricks solid into acting as liquid
Two scientists at the University of Central Florida have discovered how to get a solid material to act like a liquid without actually turning it into liquid, potentially opening a new world of possibilities for the electronic, optics and computing industries.
When chemistry graduate student Demetrius A. Vazquez-Molina took COF-5, a nano sponge-like, non-flammable manmade material and pressed it into pellets the size of a pinkie nail, he noticed something odd when he looked at its X-ray diffraction pattern. The material’s internal crystal structure arranged in a strange pattern. He took the lab results to his chemistry professor Fernando Uribe-Romo, who suggested he turn the pellets on their side and run the X-ray analysis again.
The result: The crystal structures within the material fell into precise patterns that allow for lithium ions to flow easily - like in a liquid.
The findings, published in the Journal of the American Chemical Society earlier this summer, are significant because a liquid is necessary for some electronics and other energy uses. But using current liquid materials sometimes is problematic.
Post link