#perovskites
Supercomputer predicts optical and thermal properties of complex hybrid materials
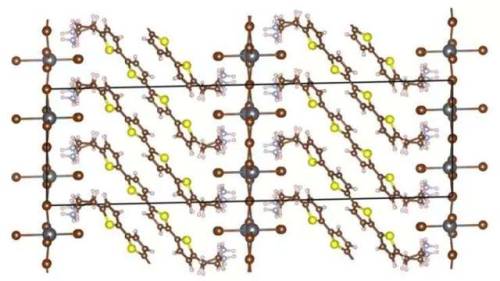
Materials scientists at Duke University computationally predicted the electrical and optical properties of semiconductors made from extended organic molecules sandwiched by inorganic structures.
These types of so-called layered “hybrid organic-inorganic perovskites"—or HOIPs—are popular targets for light-based devices such as solar cells and light-emitting diodes (LEDs). The ability to build accurate models of these materials atom-by-atom will allow researchers to explore new material designs for next-generation devices.
The results appeared online on October 4 in Physical Review Letters.
"Ideally we would like to be able to manipulate the organic and inorganic components of these types of materials independently and create semiconductors with new, predictable properties,” said David Mitzi, the Simon Family Professor of Mechanical Engineering and Materials Science at Duke. “This study shows that we are able to match and explain the experimental properties of these materials through complex supercomputer simulations, which is quite exciting.”
Post link
New Way to Power Up Nanomaterials to Create Better Solar Cells and LEDs
UCLA materials scientists and colleagues have discovered that perovskites, a class of promising materials that could be used for low-cost, high-performance solar cells and LEDs, have a previously unutilized molecular component that can further tune the electronic property of perovskites.
Named after Russian mineralogist Lev Perovski, perovskite materials have a crystal-lattice structure of inorganic molecules like that of ceramics, along with organic molecules that are interlaced throughout. Up to now, these organic molecules appeared to only serve a structural function and could not directly contribute to perovskites’ electronic performance.
Led by UCLA, a new study shows that when the organic molecules are designed properly, they not only can maintain the crystal lattice structure, but also contribute to the materials’ electronic properties. This discovery opens up new possibilities to improve the design of materials that will lead to better solar cells and LEDs. The study detailing the research was recently published in Science.
Post link
Digitally programmable perovskite nanowire-block copolymer composites
One-dimensional nanomaterials with highly anisotropicoptoelectronic properties can be used within energy harvesting applications, flexible electronics and biomedical imaging devices. In materials science and nanotechnology, 3-D patterning methods can be used to precisely assemble nanowires with locally controlled composition and orientation to allow new optoelectronic device designs. In a recent report, Nanjia Zhou and an interdisciplinary research team at the Harvard University, Wyss Institute of Biologically Inspired Engineering, Lawrence Berkeley National Laboratory and the Kavli Energy Nanoscience Institute developed and 3-D printed nanocomposite inks composed of brightly emitting colloidal cesium lead halide perovskite (CsPbX3, where X= Cl, Br, or I) nanowires.
They suspended the bright nanowires in a polystyrene-polyisoprene-polystyrene block copolymer matrix and defined the nanowire alignment using a programmed print path. The scientist produced optical nanocomposites that exhibited highly polarized absorption and emission properties. To highlight the versatility of the technique they produced several devices, including optical storage, encryption, sensing and full color displays. The work is now published on Science Advances.
Post link
New record in lead-free halide double perovskites
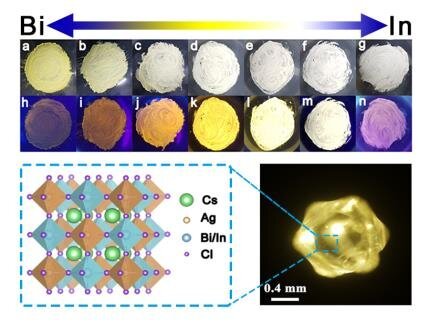
Illumination consumes more than 20 percent of electricity. Thus, finding an efficient, stable, single-phase warm white-light material is very important. Lead hybrid perovskites have drawn interest for excellent photoelectric performance and simple synthesis. Lead perovskites with white-light emission have been studied, but photoluminescence quantum efficiencies (PLQEs) are low. However, the large-scale application of lead perovskites is hindered by toxicity and instability. Therefore, the substitution of Pb with less toxic or non-toxic elements and the replacement of organic cations with relatively stable inorganic cations is being investigated.
Very recently, Keli Han’s group at the State Key Laboratory of Molecular Reaction Dynamics, Dalian Institute of Chemical Physics, Chinese Academy of Science, reports a series of bulk lead-free double perovskites: Cs2AgBi1-xInxCl6 (0 < x < 1). They demonstrate the existence of the parity-forbidden transition by photophysical characterization in Cs2AgInCl6 bulk crystal. The Cs2AgBi0.125In0.875Cl6 breaks the parity-forbidden transition and shows warm white-light emission with broad emission across the entire visible spectrum, with the highest PLQE of 70.3%.
Post link
Revealing the hidden path of perovskite formation
Perovskite solar cells are an alternative to conventional silicon solar cells, poised to enter the market with their high power-conversion efficiencies (above 22% now) and lower capital expenditure and manufacturing costs.
One of the main methods for depositing perovskite films onto panel structures is a process known as sequential deposition reaction, which was developed in 2013 by Michael Grätzel and co-workers at EPFL. Many studies have attempted to control this process with additives, compositional changes, and temperature effects. However, none of these has provided a complete understanding of the entire sequential deposition reaction. This prevents adequate control over film quality, which determines the performance of the solar cell.
A study by Michael Grätzel and Amita Ummadisingu at EPFL now offers the most systematic and full study of the sequential deposition reaction to-date. The scientists began with X-ray diffraction analysis and scanning electron microscopy to study in depth the crystallization of lead iodide (PbI2), which is the first stage of the reaction. They then used, for the first time, SEM-cathodoluminescence imaging to study the nano-scale dynamics of perovskite film formation.
“We have combined two powerful tools to obtain compositional information about the surface of the film during perovskite formation,” says Amita Ummadisingu. “This technique enables us to achieve stunning nano-scale resolution meaning that we can see, for the first time, that mixed crystalline aggregates composed of perovskite and PbI2 are formed during the reaction.”
Post link
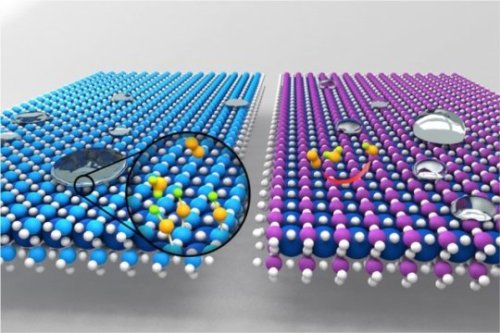
How to look for a few good catalysts
Two key physical phenomena take place at the surfaces of materials: catalysis and wetting. A catalyst enhances the rate of chemical reactions; wetting refers to how liquids spread across a surface.
Now researchers at MIT and other institutions have found that these two processes, which had been considered unrelated, are in fact closely linked. The discovery could make it easier to find new catalysts for particular applications, among other potential benefits.
“What’s really exciting is that we’ve been able to connect atomic-level interactions of water and oxides on the surface to macroscopic measurements of wetting, whether a surface is hydrophobic or hydrophilic, and connect that directly with catalytic properties,” says Yang Shao-Horn, the W.M. Keck Professor of Energy at MIT and a senior author of a paper describing the findings in the Journal of Physical Chemistry C. The research focused on a class of oxides called perovskites that are of interest for applications such as gas sensing, water purification, batteries, and fuel cells.
Since determining a surface’s wettability is “trivially easy,” says senior author Kripa Varanasi, an associate professor of mechanical engineering, that determination can now be used to predict a material’s suitability as a catalyst. Since researchers tend to specialize in either wettability or catalysis, this produces a framework for researchers in both fields to work together to advance understanding, says Varanasi, whose research focuses primarily on wettability; Shao-Horn is an expert on catalytic reactions.
Post link
Adding cesium to perovskite in solar cells boosts performance of silicon
A team of researchers working at Oxford University has found a way to add cesium to perovskite solar cells to boost the performance of silicon, while maintaining the efficiency benefits it offers. In their paper published in the journal Science, the team describes their process which included finding a way to overcome the problem of efficiency loss in such materials that normally come about due to a limited range of solar spectrum use.
As researchers around the world continue to look for the next-generation material to use for solar power collection to increase efficiency, others continue to seek ways to improve the standard now in use: silicon. In this new effort the research team noted the work done by others looking into the possibility of using perovskites (minerals made mostly of calcium titanate) as possible replacements for silicon, and found a way to add cesium to the mineral to make it work in tandem with silicon to create a solar collector that is up to 25 percent more efficient than those now in use. Such an improvement in performance could signal a transformation in real world use—solar power has thus far not proven to be efficient enough for the average consumer to cut the cord from the utility company—doubling efficiency might just make doing so a smart investment.
Up until now, efforts to get perovskites to work in tandem with silicon have been held back by inefficiencies in the cells due to the range of solar spectrum they were able to use—attempts to tweak the mix have led to instability in the materials. To overcome this problem, the team at Oxford came up with a process based on substituting certain ions in the material with cesium ions—it solved the spectrum problem, they report, while maintaining the stability of the overall structure.
Post link
Materials research team lights the way for more efficient LEDs
Researchers at the U.S. Naval Research Laboratory (NRL) Center for Computational Materials Science, working with an international team of physicists, have revealed that nanocrystals made of cesium lead halide perovskites (CsPbX3), is the first discovered material which the ground exciton state is “bright,” making it an attractive candidate for more efficient solid-state lasers and light emitting diodes (LEDs).
“The discovery of such material, and understanding of the nature of the existence of the ground bright exciton, open the way for the discovery of other semiconductor structures with bright ground excitons,” said Dr. Alexander Efros, research physicist, NRL. “An optically active bright exciton in this material emits light much faster than in conventional light emitting materials and enables larger power, lower energy use, and faster switching for communication and sensors.”
The work, which was partially sponsored by the Office of Naval Research through a program managed by Dr. Chagaan Baatar, studied lead halide perovskites with three different compositions, including chlorine, bromine, and iodine. Nanocrystals made of these compounds and their alloys can be tuned to emit light at wavelengths that span the entire visible range, while retaining the fast light emission that gives them their superior performance.
Post link
Aberrant electronic and structural alterations in pressure-tuned perovskite
The perovskite NaOsO3 has a complicated but interesting temperature-dependent metal-insulator transition (MIT). A team led by Drs. Raimundas Sereika and Yang Ding from the Center for High Pressure Science and Technology Advanced Research (HPSTAR) showed that the insulating ground state in NaOsO3 can be preserved up to at least 35 GPa with a sluggish MIT reduction from 410 K to a near room temperature and possible transformation to a polar phase. The work has been published in npj Quantum Materials.
NaOsO3 perovskite undergoes a metal-insulator transition concomitant with the onset of an antiferromagnetic long-range ordering at a Neel temperature of about 410 K, which is accompanied by a magnetic ordering without any lattice distortion.
The team carried out a combined experimental and computational study to understand the effect of external pressure on perovskite NaOsO3. They found hidden hysteretic resistance properties with a transient metallic state near 200 K. Also three electronic character anomalies (at 1.7, 9.0, and 25.5 GPa), and a structural transition to the singular polar phase (at ~ 18 GPa) were discovered.
Post link
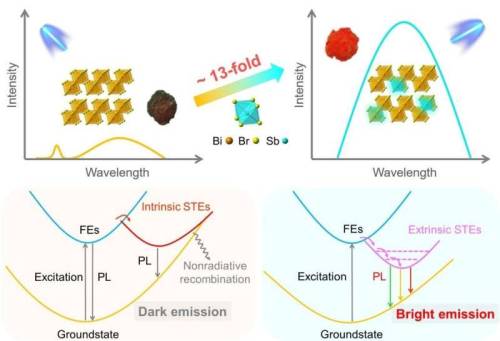
Developing inorganic lead-free perovskite for broadband emission
Artificial lighting accounts for one-fifth of global electricity consumption, and developing efficient and stable luminescence materials is critical to avoid unnecessary waste of electric energy. The single emitters with broadband emission, such as lead halide perovskites, have recently triggered tremendous attention for artificial illumination and display applications. To develop lead-free and stable perovskites with broadband emission, researchers in China targeted low-dimensional bismuth halide perovskites.
They published their work on Apr. 15 in Energy Material Advances.
“The single emitters with broadband emission can circumvent critical problems faced in the traditional mixed and multicomponent emitters such as the efficiency losses caused by self-absorption, the complex device structure, and the colors instability due to the different degradation rates of phosphors,” said paper author Rengui Li, a professor with the State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS). “Lead halide perovskites have emerged as highly attractive next-generation optoelectronic materials for light-emitting applications due to their extraordinary photoelectric properties.”
Post link
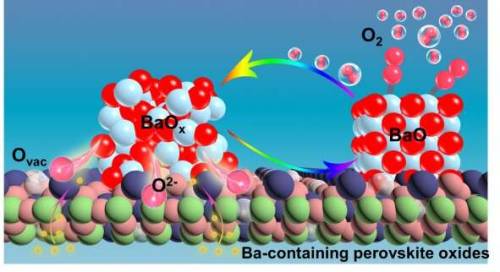
Mechanism of oxygen activation on barium-containing perovskite materials
A research team led by Prof. Yang Weishen and Prof. Zhu Xuefeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed the mechanism of oxygen activation on Barium-containing perovskite materials.
The researchers discovered that BaO/BaO2 nanoparticles precipitated on the surface of Ba-containing materials under high-temperature oxygen-rich conditions had an ultra-high activity for oxygen activation, which clarified the mechanism of high-temperature oxygen activation and transport on the surface of Ba-containing perovskite oxides.
This study was published in Science Advances on April 13.
In 2000, the DICP team invented an oxygen-permeable membrane material named Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF). Due to its good catalytic activity towards oxygen activation, BSCF has become a representative material for oxygen permeation and has been widely used in solid oxide fuel cells, oxygen reduction reactions, and oxygen evolution reactions.
Post link
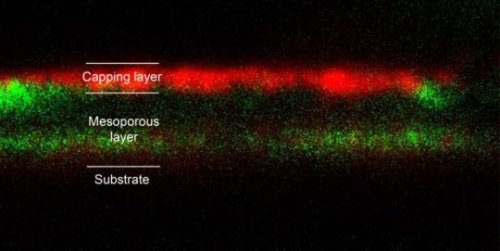
Imploding bubbles improve the growth of single-crystalline perovskite solar cell thin films
Single thin-film crystals are required to utilize promising hybrid organic/inorganic perovskite materials to develop solar cells. Saudi Arabia’s King Abdullah University of Science and Technology (KAUST) researchers have now shown how imploding bubbles in a solution can grow single crystals of the preferred orientation for manufacturing thin films.
Hybrid organic/inorganic perovskite materials are some of the most promising solar cell materials, as they are easy to produce and show excellent solar light conversion efficiencies.
“Our monocrystalline films provide a platform to directly implement perovskite single crystals and to elucidate their promises and challenges for solar cells,” said Osman Bakr, KAUST associate professor of material science and engineering who also led the research study.
Hybrid perovskite materials can easily be fabricated on a large scale from a solvent solution. However, this process typically produces polycrystalline perovskite films that have a smaller solar light conversion efficiency than monocrystalline materials because the boundary between crystalline grains leads to losses. In particular, growth of single-crystalline perovskite solar cell films has not yet been achieved on top of other materials, which is a requirement for practical devices.
Post link
A team of physicists has defied conventional wisdom by inducing stable ferroelectricity in a sheet of strontium titanate only a few nanometers thick. The discovery could open new pathways to find new materials for nanotechnology devices, said Alexei Gruverman, a University of Nebraska-Lincoln…

A sample all-inorganic perovskite solar cell is a step towards toward commercial use, according to scientists at Rice University. Their discovery of a way to quench defects in cesium-lead-iodide solar cells allowed them to preserve the material’s band gap. Image credit: Jeff Fitlow/Rice University
By Anthony Caggiano
Rice University scientists have replaced some of the lead in perovskite solar cells with indium, which could help improve their performance.
Rice materials scientist Jun Lou and his colleagues at the Brown School of Engineering have been able to better manage defects in cesium-lead-iodide solar cells that affect the compound’s band gap, a critical property in solar cell efficiency.
The cells can also be made in open air and last for months rather than days with a solar conversion efficiency slightly above 12%.
Traditionally, materials used in perovskites - crystals with cube-like lattices and are efficient light harvesters – tend to be stressed by light, humidity and heat.
Rice postdoctoral researcher and lead author Jia Liang and his team built and tested perovskite solar cells of inorganic cesium, lead and iodide, the very cells that tend to fail quickly due to defects. But by adding bromine and indium, the researchers were able to quash defects in the material, raising the efficiency above 12% and the voltage to 1.20 volts.
As a bonus, the material proved to be exceptionally stable. The cells were prepared in ambient conditions, standing up to Houston’s high humidity, and encapsulated cells remained stable in air for more than two months, far better than the few days that plain cesium-lead-iodide cells lasted.
‘The highest efficiency for this material may be about 20%, and if we can get there, this can be a commercial product,’ Liang said. ‘It has advantages over silicon-based solar cells because synthesis is very cheap, it’s solution-based and easy to scale up. Basically, you just spread it on a substrate, let it dry out, and you have your solar cell.’