#antibiotic resistance
Researchers identify a bacteria on healthy cats that produces antibiotics against severe skin infections; the findings may lead to new bacteriotherapies for humans and their pets
Researchers at University of California San Diego School of Medicine used bacteria found on healthy cats to successfully treat a skin infection on mice. These bacteria may serve as the basis for new therapeutics against severe skin infections in humans, dogs and cats.
The study, published in eLife on October 19, 2021, was led by Richard L. Gallo, MD, PhD, Distinguished Professor and chair of the Department of Dermatology at UC San Diego School of Medicine, whose team specializes in using bacteria and their products to treat illnesses — an approach known as “bacteriotherapy.”
Skin is colonized by hundreds of bacterial species that play important roles in skin health, immunity and fighting infection. All species need to maintain a diverse balance of healthy skin bacteria to fight potential pathogens.
“Our health absolutely depends on these ‘good’ bacteria,” said Gallo. “They rely on our healthy skin to live, and in return some of them protect us from ‘bad’ bacteria. But if we get sick, ‘bad’ bacteria can take advantage of our weakened defenses and cause infection.”
This is the case with methicillin-resistant Staphylococcus pseudintermedius (MRSP), a bacterium commonly found on domesticated animals that becomes infectious when the animals are sick or injured. MRSP is an emerging pathogen that can jump between species and cause severe atopic dermatitis, or eczema. These infections are common in dogs and cats, and can also occur in humans, though rates of human infection vary around the world. As its name suggests, MRSP is resistant to common antibiotics and has been difficult to treat in clinical and veterinary settings.
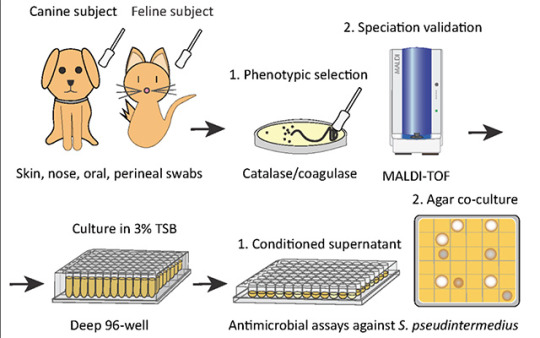
To address this, researchers first screened a library of bacteria that normally live on dogs and cats and grew them in the presence of MRSP. From this, they identified a strain of cat bacteria called Staphylococcus felis(S. felis) that was especially good at inhibiting MRSP growth. They found that this special strain of S. felis naturally produces multiple antibiotics that kill MRSP by disrupting its cell wall and increasing the production of toxic free radicals.
“The potency of this species is extreme,” said Gallo. “It is strongly capable of killing pathogens, in part because it attacks them from many sides — a strategy known as ‘polypharmacy.’ This makes it particularly attractive as a therapeutic.”
Bacteria can easily develop resistance to a single antibiotic. To get around this, S. felis has four genes that code for four distinct antimicrobial peptides. Each of these antibiotics is capable of killing MRSP on their own, but by working together, they make it more difficult for the bacteria to fight back.
Having established how S. felis kills the MRSP, the next step was to see whether it could work as a therapy on a live animal. The team exposed mice to the most common form of the pathogen and then added either S. felis bacteria or bacterial extract to the same site. The skin showed a reduction in scaling and redness after either treatment, compared with animals that had no treatment. There were also fewer viable MRSP bacteria left on the skin after treatment with S. felis.
Next steps include plans for a clinical trial to confirm whether S. felis can be used to treat MRSP infections in dogs. Bacteriotherapies like this one can be delivered via topical sprays, creams or gels that contain either live bacteria or purified extract of the antimicrobial peptides.
Pictured above: To identify candidates for a new bacteriotherapy against skin infection, researchers first screened various bacteria from dogs and cats and co-cultured them with S. pseudintermedius in liquid and agar antimicrobial assays.
“Cat Bacteria Treats Mouse Skin Infection, May Help You and Your Pets As Well”
A team of doctors and researchers working at Erasmus Hospital in Belgium has successfully treated an adult woman infected with a drug-resistant bacteria using a combination of bacteriophage therapy and antibiotics. In their paper published in the journal Nature Communications, the group describes the reasons for the use of the treatment and the ways it might be used in other cases.
Bacteriophages are viruses that infect and kill bacteria. Research involving their use in human patients has been ongoing for several decades, but they are still not used to treat patients. In this new effort, the researchers were presented with a unique opportunity not only to treat a patient in need of help, but to learn more about the possible use of viruses to treat patients infected with bacteria that have become resistant to conventional antibiotics.
Bacteria may Demonstrate any of Five General Mechanisms of Antibiotic Resistance:
1. Lack of entry; Decreased cell permeability.
2. Greater exit; Active efflux.
3. Enzymatic inactivation of the antibiotic.
4. Altered target; Modification of drug receptor site.
5. Synthesis of resistant metabolic pathway.
More than HIV, more than malaria. The death toll worldwide from bacterial antimicrobial resistance (AMR) in 2019 exceeded 1.2 million people, according to a new study.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Check out this time-lapse video that shows a superbug, once thought of as static or non-motile, exhibiting signs of active motility.
Scientists can prove, for the first time, that S. aureus may be able to move independently, which could have major implications for treating future infections caused by the once difficult-to-treat, antibiotic-resistant MRSA.
