#microbiome
Gut Bacteria May Play a Role in Autism (Scientific American)
Autism is primarily a disorder of the brain, but research suggests that as many as nine out of 10 individuals with the condition also suffer from gastrointestinal problems such as inflammatory bowel disease and “leaky gut.” The latter condition occurs when the intestines become excessively permeable and leak their contents into the bloodstream. Scientists have long wondered whether the composition of bacteria in the intestines, known as the gut microbiome, might be abnormal in people with autism and drive some of these symptoms. Now a spate of new studies supports this notion and suggests that restoring proper microbial balance could alleviate some of the disorder’s behavioral symptoms.
At the annual meeting of the American Society for Microbiology held in May in Boston, researchers at Arizona State University reported the results of an experiment in which they measured the levels of various microbial by-products in the feces of children with autism and compared them with those found in healthy children. The levels of 50 of these substances, they found, significantly differed between the two groups. And in a 2013 study published in PLOS ONE, Italian researchers reported that, compared with healthy kids, those with autism had altered levels of several intestinal bacterial species, including fewer Bifidobacterium, a group known to promote good intestinal health.
One open question is whether these microbial differences drive the development of the condition or are instead a consequence of it. A study published in December 2013 in Cell supports the former idea. When researchers at the California Institute of Technology incited autismlike symptoms in mice using an established paradigm that involved infecting their mothers with a viruslike molecule during pregnancy, they found that after birth, the mice had altered gut bacteria compared with healthy mice. By treating the sick rodents with a health-promoting bacterium called Bacteroides fragilis, the researchers were able to attenuate some, but not all, of their behavioral symptoms. The treated mice had less anxious and stereotyped behaviors and became more vocally communicative.
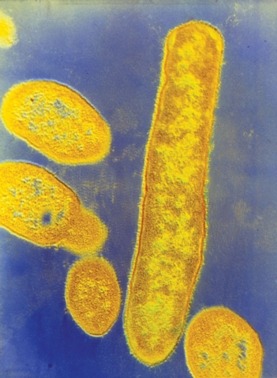
Bacteroides fragilis
Credit: CNRI/SCIENCE SOURCE
Post link
Too many antibiotics can give preemies a lifetime of ill health
Today, babies born as early as 28 weeks routinely survive, as do more than half of those born at 24 weeks (although often with significant disabilities). Much of the credit goes to antibiotics, which have thwarted infections such as sepsis and group B strep that a preemie’s immature immune system could not have fought on its own. Those successes spurred a steady increase in routine antibiotic use in the NICU. At last count, three of the top four drugs prescribed in the NICU were antibiotics.
Over time, however, scientists began noticing that antibiotics can increase babies’ risk of the very afflictions the drugs aim to protect against—such as fungal infections, late-onset sepsis, and a deadly intestinal disorder called necrotizing enterocolitis. In a seminal 2009 study in Pediatrics, for example, Greenberg’s colleague Michael Cotten showed that each additional day of antibiotics significantly increased the odds that a preemie would develop necrotizing enterocolitis or die.
Researchers are still debating when the first microbes colonize us—in utero or during birth—but Greenberg and many others worry that early use of antibiotics in infants disrupts the establishment of those indispensable residents. The gut microbiome is practically an organ unto itself, weighing about as much as the liver. It is thought to play a critical role in priming the immune system, and it produces just as many neurotransmitters as the human brain. Genetic and environmental factors, including antibiotics, shape its makeup early in life. Then, around age 3, a quasi-stability sets in and we are “stuck with that architecture,” says Gautam Dantas, a microbiologist at Washington University in St. Louis, Missouri.
Post link

IMAGE: © iStock Photo / royaltystockphoto
An industrial chemical — phased out since 2002, but previously used in stain and water-repellent products and firefighting foam — alters the gut microbiome of mice and could have implications for human health, according to an international team of researchers.
Perfluorooctane sulfonate, or PFOS, persists in the environment and in the bodies of living organisms. While the U.S. Environmental Protection Agency designated PFOS a “contaminant of emerging concern” and its production was voluntarily ceased in the United States by producers, it is still detected in the blood of up to 99% of the U.S. population.
“We know that chronic exposure to some environmental chemicals, including persistent organic pollutants, can impact the gut microbiome, and we are actively assessing whether these interactions can impact health,” said Andrew Patterson, Tombros Early Career Professor and professor of molecular toxicology, Penn State. “Our study shows that PFOS alters the composition and function of the microbiome, which suggests that this chemical and perhaps related chemicals, have mechanisms of action outside our own cells. Exploring how chemicals impact the microbiome is an important and emerging area of study.”
Read more about this work at Penn State News!
State Turtle legislation was fast tracked in New Jersey and beat out Streptomyces griseus to become the newest State Symbol.
Microbiologists are dismayed, far and wide.
Although the bog turtle never saved anyone’s life, (unlike the microbe) they are visible without a microscope and had the support of school kids.
Get a great microbiology book: https://tinyurl.com/Warhol-Small-Guide
Post link
NJ State Microbe moving ahead again!
On June 14, the NJ Senate State Government. Wagering, Tourism & Historic Preservation Committee voted Yes unanimously on S1729, which names Streptomyces griseus as the State Microbe of New Jersey. This is another big step in becoming the first state after Oregon to have an official microbe, and an excellent way of acknowledging the great science that’s been done in NJ.
Thank you Senators!
Thank you scientists for writing in!
Twitter @WarholScience
Get the best new microbiology book:https://tinyurl.com/Warhol-Small-Guide
Post link
A Milestone. Thanks!
Please check out the book https://tinyurl.com/Warhol-Small-Guide
Twitter @WarholScience
Post link
Microbial School Outreach
Rutgers Professor Dr Jeff Boyd took time from his busy schedule to talk about microbes with students at Bartle Elementary School in Highland Park, NJ. He spoke about all manner of invisible life forms, but really got the kids enthused about the role of Streptomyces griseus in antibiotic production and improving healthcare worldwide.
Dr Boyd also wrote to Assemblywoman Pinkin (18th District) who was so impressed with the Strep griseus story that she promised to vote for A3650, the New Jersey State Microbe law.
A great book for microbiologists and non-microbiologists https://tinyurl.com/Warhol-Small-Guide
Post link
Quiero comenzar con esta nueva etapa para la cuenta compartiendo con ustedes unas notas rápidas sobre un artículo muy interesante sobre “El microbioma en la piel humana”
I want to start this new stage for the account by sharing with you some quick notes about a very interesting article about “The microbiome in human skin”




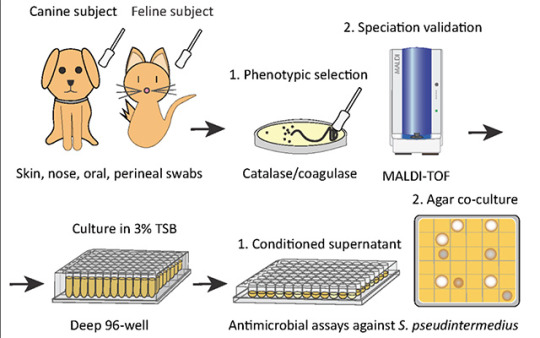
Researchers identify a bacteria on healthy cats that produces antibiotics against severe skin infections; the findings may lead to new bacteriotherapies for humans and their pets
Researchers at University of California San Diego School of Medicine used bacteria found on healthy cats to successfully treat a skin infection on mice. These bacteria may serve as the basis for new therapeutics against severe skin infections in humans, dogs and cats.
The study, published in eLife on October 19, 2021, was led by Richard L. Gallo, MD, PhD, Distinguished Professor and chair of the Department of Dermatology at UC San Diego School of Medicine, whose team specializes in using bacteria and their products to treat illnesses — an approach known as “bacteriotherapy.”
Skin is colonized by hundreds of bacterial species that play important roles in skin health, immunity and fighting infection. All species need to maintain a diverse balance of healthy skin bacteria to fight potential pathogens.
“Our health absolutely depends on these ‘good’ bacteria,” said Gallo. “They rely on our healthy skin to live, and in return some of them protect us from ‘bad’ bacteria. But if we get sick, ‘bad’ bacteria can take advantage of our weakened defenses and cause infection.”
This is the case with methicillin-resistant Staphylococcus pseudintermedius (MRSP), a bacterium commonly found on domesticated animals that becomes infectious when the animals are sick or injured. MRSP is an emerging pathogen that can jump between species and cause severe atopic dermatitis, or eczema. These infections are common in dogs and cats, and can also occur in humans, though rates of human infection vary around the world. As its name suggests, MRSP is resistant to common antibiotics and has been difficult to treat in clinical and veterinary settings.
To address this, researchers first screened a library of bacteria that normally live on dogs and cats and grew them in the presence of MRSP. From this, they identified a strain of cat bacteria called Staphylococcus felis(S. felis) that was especially good at inhibiting MRSP growth. They found that this special strain of S. felis naturally produces multiple antibiotics that kill MRSP by disrupting its cell wall and increasing the production of toxic free radicals.
“The potency of this species is extreme,” said Gallo. “It is strongly capable of killing pathogens, in part because it attacks them from many sides — a strategy known as ‘polypharmacy.’ This makes it particularly attractive as a therapeutic.”
Bacteria can easily develop resistance to a single antibiotic. To get around this, S. felis has four genes that code for four distinct antimicrobial peptides. Each of these antibiotics is capable of killing MRSP on their own, but by working together, they make it more difficult for the bacteria to fight back.
Having established how S. felis kills the MRSP, the next step was to see whether it could work as a therapy on a live animal. The team exposed mice to the most common form of the pathogen and then added either S. felis bacteria or bacterial extract to the same site. The skin showed a reduction in scaling and redness after either treatment, compared with animals that had no treatment. There were also fewer viable MRSP bacteria left on the skin after treatment with S. felis.
Next steps include plans for a clinical trial to confirm whether S. felis can be used to treat MRSP infections in dogs. Bacteriotherapies like this one can be delivered via topical sprays, creams or gels that contain either live bacteria or purified extract of the antimicrobial peptides.
Pictured above: To identify candidates for a new bacteriotherapy against skin infection, researchers first screened various bacteria from dogs and cats and co-cultured them with S. pseudintermedius in liquid and agar antimicrobial assays.
“Cat Bacteria Treats Mouse Skin Infection, May Help You and Your Pets As Well”
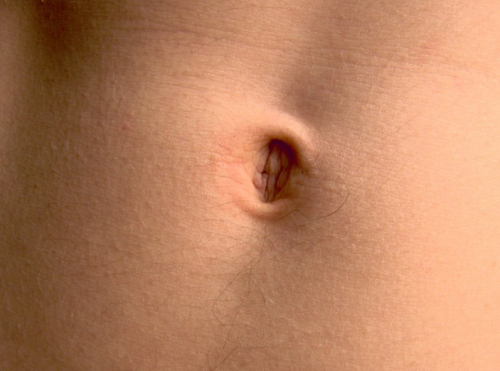
Did you know? Your belly button is home to much, much more than just the occasional piece of lint.
In 2012, researchers observed spectacular microbial diversity in belly buttons—in 60 different belly buttons surveyed, they found a total of nearly 2,400 phylotypes (a phylotype is shorthand in microbiome studies for “species”) of bacteria. The vast majority of these phylotypes (2,188) were found in fewer than 10 percent of the belly buttons studied. Even though there were 200 or so phylotypes found in more than 10 percent of the belly buttons, none of these were found in all belly buttons, and only eight phylotypes are found in more than 70 percent of the belly buttons. The authors of the work suggest that the belly button biodiversity is on par with the animal diversity in a jungle!
Post link
Why is a mouth like a coral reef?
No, this isn’t a nonsense riddle form Lewis Carroll. But it is the subject of the most recent episode of This Week in Virology (TWiV), where host Vincent Racaniello talks with guests David Pride and Forest Rohwer about their work in the microbiomes of human mouths and the viromes of coral reefs. These two worlds have more in common than you might think when it comes to viruses and bacteria!
“Biology is really all mucus,” (10:09), says cohost Alan Dove, and this is certainly true of coral reefs. According to microbial ecologist Rohwer, coral has thick mucus layers that interact with the ocean and the microbes that come with it. Living inside coral are huge numbers of microorganisms, like dinoflagellates, and when different microorganisms from the water interact with them, a number of things happen. Usually, when phages meet bacteria, they constantly fight each other, but in some coral at certain densities the phages are likely to become prophages that instead protect the bacteria from other phages. The phages, with the mucus, create a kind of protective barrier.
And what about the mouth? “We’re just a coral reef turned inside out,” (12:17) says Dove, as Pride, doctor of Microbiology and Immunology, explains how the saliva microbiome washes over and interacts with the multiple microbiomes located within the mouth. These different microbiomes, called microniches, are all essentially distinct from one another, containing their own makeup of viruses. If some of the viruses are removed, as by using some antibiotics, the makeup is drastically altered, and a space is left open for many more things to come in.
Rohwer notes that the same is true for coral reefs; if phages are removed, bacteria increase and slow the system down. If you add the phages back in, the bacteria decrease again.
“If you mess with any one component of this,” says cohost Rich Condit, “you’re potentially reaching a crisis where the whole thing spins out of control and crashes” (52.48).
Post link
It’s a regular Wednesday morning, and some of you reading this might be sitting in your office, sipping coffee, just trying to get through your morning workload.
You might be sitting in a cubicle, surrounded by coworkers, or you might have your own office, but we’re here to remind you that you’re never truly alone at work - we share our office chairs and keyboards with microbes, and breathe the same office air as them! Don’t take out your sanitizer just yet, because, after all, not allmicrobes are harmful! In fact, they are a big part of our environment.
We’ve all heard of microbiomes in the gut that are specific to the individual - but now there are reported, scientific findings that show that microbiomes also exist in the office. According to studies conducted by Gregory Caporaso and his team at Northern Arizona University, every office environment has a microbiome that’s specific to its location. The specificity is the result of varied external environments.
The study was conducted in nine offices across Flagstaff, San Dieog, and Toronto, all chosen for their varied climates. Collection plates were installed in offices in each of these cities, and the plates were covered in materials such as carpet, tile, and drywall to test for microbial differences across materials.
Researchers then discovered that there were no differences in bacterial communities across materials, but that their location in a particular room did matter. Overall, samples taken from offices in the same city were more similar to one another than samples from other cities. This is mostly a result of the external environment, such as climate, vegetation, and industry.
It remains to be discovered whether some microbiomes are making us unhealthier than others. However, we could potentially use this knowledge to make our environments healthier by engineering healthier microbiomes. This technology might sound like science fiction, but, then again, just 15 years ago, so did smartphones!
Read more about cities and their unique microbes here.
Post link
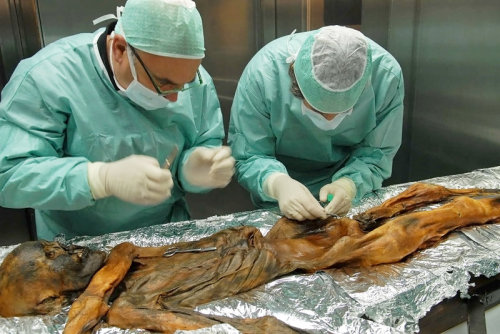
#TBT: Researchers have found, within the stomach of a prehistoric and well-preserved ice mummy (Oetzi the Iceman, to be exact), remains of his once-thriving gut bacteria! This means that the human microbiome has existed for millenia.
Although Oetzi’s been in the hands of scientists since 1991, researchers have only just now ventured into his stomach (largely because it was so shriveled they’ve had some trouble getting to it!), finding that it is “full of material.” In particular, they found what was presumably his last supper (meat from a deer and an alpine mountain goat), as well as DNA from a type of bacteria that lived in his gut - Helicobacter pylori.
According to a physician and microbiologist not related to the study, “Today, H. pylori can be found in about half of the world’s population. In people who have it, it’s the dominant organism in their stomach.”
WhileH. pylori has been known as a harmful pathogen, causing ulcers and stomach cancers, it also provides some benefits, such as protecting against acid reflux and asthma. The bacterium is probably most beneficial to scientists, who use “its evolution to map the paths of ancient populations as they moved across the globe.”
Read up on Oetzi (an interesting guy, really - he suffered from parasitic worms, Lyme disease, tooth decay, and joint problems, among other ailments, he has at least 19 living relatives somewhere in Austria, and he had 60-plus tattoos) and his gut bacteria at NPRorThe Atlantic.
Post link
All caught up on Making a Murderer and looking for more? Then read up on a new study published last month in Scienceabout how microbes can help solve a murder mystery.
Jessica Metcalf of University of Boulder, Colorado and her team have shown that the cadaver microbes (a “dedicated coterie of bacteria, fungi, and nematode worms” that feast on the nutrients from a corpse) - a.k.a. the necrobiome - change in such a predictable way that forensic investigators can tell how long ago that person died.
According to Metcalf, your microbes are “like witnesses to your death. As you decompose, they can help investigators solve your murder.” In addition to helping investigators figure out when someone died, the victim’s microbes can also offer clues as to wherethey died - information that could be used to track the movements of the suspect(s).
Microbes left behind from the perp - similar to fingerprints - can also help solve the crime, by determining whether someone handled a murder weapon or other objects at the scene of the crime.
Read more at The Atlantic, or listen to the All Things Considered story.
Post link
Do you ever think about where you leave your DNA? Here’s one place you may not have considered: an ATM. NYU researchers took swabs from 66 ATMs around Manhattan, Queens, and Brooklyn, and found that the keypads on automated teller machines reveal traces of microbes from human skin as well as from food and household surfaces like kitchens, pillows, and yes, bathrooms. This means that ATMs are a great source for mapping the city’s DNA.
From Science Daily:
“Our results suggest that ATM keypads integrate microbes from different sources, including the human microbiome, foods, and potentially novel environmental organisms adapted to air or surfaces,“ explains senior study author Jane Carlton, director of the Center for Genomics and Systems Biology and professor of biology at NYU. "DNA obtained from ATM keypads may therefore provide a record of both human behavior and environmental sources of microbes.”
In addition, researchers found that certain biomarkers were more common depending on where the ATM was located, such as more Lactobacillales (lactic acid bacteria) found in laundromat ATMs, while Xeromyces bisporus, found in spoiled baked goods, appeared most often in Manhattan. Because so many people use these ATMs daily, scientists are confident that these keypads represent an “average” microbial community, one that reflects both environmental and urban contact.
It also means you might want to wash your hands the next time you take out some cash.
Read more at Science Daily.
Post link
University of California researcher Bruce German has a lot to say on the subject of milk - specifically, human breast milk. While all mammals produce milk, the makeup of the substance differs from species to species, as one might expect, to cater to that species’ needs. But in researching the composition of human milk, German found a surprise.
From the New Yorker:
Every mammal mother produces complex sugars called oligosaccharides, but human mothers, for some reason, churn out an exceptional variety: so far, scientists have identified more than two hundred human milk oligosaccharides, or H.M.O.s. They are the third-most plentiful ingredient in human milk, after lactose and fats, and their structure ought to make them a rich source of energy for growing babies—but babies cannot digest them. When German first learned this, he was gobsmacked. Why would a mother expend so much energy manufacturing these complicated chemicals if they were apparently useless to her child?
The answer, researchers found, is that these H.M.O.s travel into the large intestine, where they are fed upon by a microbe called Bifidobacterium longum infantis, which will cause them to grow faster and more efficiently than any other gut bacteria. This process is motivated by evolution, and is beneficial to both the bacteria and the child.
As [B. infantis] digests H.M.O.s, it releases short-chain fatty acids, which feed an infant’s gut cells. Through direct contact, B. infantis also encourages gut cells to make adhesive proteins that seal the gaps between them, keeping microbes out of the bloodstream, and anti-inflammatory molecules that calibrate the immune system. These changes only happen when B. infantis feeds on H.M.O.s; if it gets lactose instead, it survives but doesn’t engage in any repartee with the baby’s cells. In other words, the microbe’s full beneficial potential is unlocked only when it feeds on breast milk.
There are a number of other ideas as to why such a vast and diverse number of H.M.O.s exist in humans over other mammals. Some scientists suggest that feeding B. infantis helps to stimulate brain growth; others note that it serves as a defense against pathogens. At a pediatric hospital in California, these microbes might be a solution to help nourish premature infants.
To learn more about H.M.O.s and Bifidobacteria, visit the full New Yorker article here.
Post link
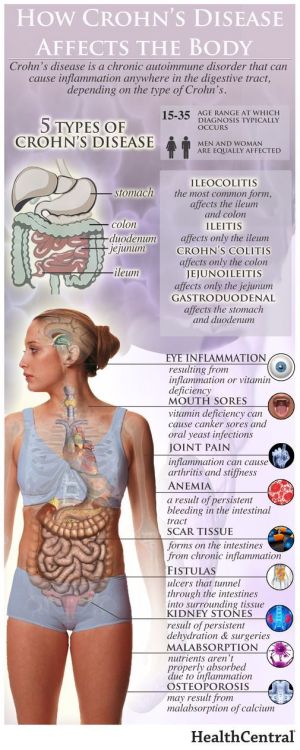
Case Western Reserve University School of Medicine has identified a fungus that is a key factor in Crohn’s disease. There is hope that this discovery, along with their finding of a new bacterium that is linked to the previous bacteria associated with the disease, will lead to new treatments and a cure.
From Science Daily:
“We already know that bacteria, in addition to genetic and dietary factors, play a major role in causing Crohn’s disease,” said the study’s senior and corresponding author, Mahmoud A Ghannoum, PhD, professor and director of the Center for Medical Mycology at Case Western Reserve and University Hospitals Cleveland Medical Center “Essentially, patients with Crohn’s have abnormal immune responses to these bacteria, which inhabit the intestines of all people. While most researchers focus their investigations on these bacteria, few have examined the role of fungi, which are also present in everyone’s intestines. Our study adds significant new information to understanding why some people develop Crohn’s disease.
The research found that the combined presence of the two bacteria, Escherichia coli and Serratia marcescens, and the fungi Candida tropicalis was much higher in fecal samples of people with Crohn’s compared to those without the disease.
Test-tube research by the Ghannoum-led team found that the three work together (with the E. coli cells fusing to the fungal cells and S. marcescens forming a bridge connecting the microbes) to produce a biofilm – a thin, slimy layer of microorganisms found in the body that adheres to, among other sites, a portion of the intestines – which can prompt inflammation that results in the symptoms of Crohn’s disease.
To learn more about the first time a fungus has been linked to Crohn disease in humans, read more at Science Daily or find the research here through Case Western.
Post link
An unlikely superhero may have been identified in the fight against superbug MRSA–– the human nose! Noses often contain Staphylococcus aureus, but apparently also contain a compound that acts as a natural enemy of MRSA (the methicillin-resistant strain of S. aureus.)
Scientists at University of Tübingen in Germany isolated this nasal bacterium, S. lugdunensis, and discovered that it destroyed S. aureus in a petri dish when grown together.
From ScienceNews.org: “We didn’t expect to find this. We were just trying to understand the ecology of the nose to understand how S. aureus causes problems,” bacteriologist Andreas Peschel of the University of Tübingen in Germany said at a news briefing July 26 during the EuroScience Open Forum. Investigating the intense interspecies competition in the nose — where microbes fight for space and access to scant sugars and amino acids — might offer a fertile alternative to searching for new drug candidates in soil microbes.”
Learn more about this study and the possible antibiotic implications in the article “The nose knows how to fight staph” at ScienceNews.org, or read the study “Human commensals producing a novel antibiotic impair pathogen colonization” at Nature.
Post link