#sputtering
Aluminum target for our sputter system. If you look closely you can see individual crystal grains.
Sputtering is a technique used to deposit thin films of material. The material source is called a target because it is bombarded with high energy atoms which remove bits of material that are then redeposited on your sample or wafer. The ring is a result of the magnetic field confining the plasma to that region.
Post link
Sample stage controls for an ion mill, allowing for rotation about two different axes.
Ion milling is a type of dry etch process used to remove parts of a sample by bombarding it with ions, typically argon, in a vacuum chamber. It can be thought of as an atomic sand blaster. Ions (the “grains of sand”) physically expel, or sputter, chunks of material from the surface. The sample is rotated to ensure uniform coverage.
Post link
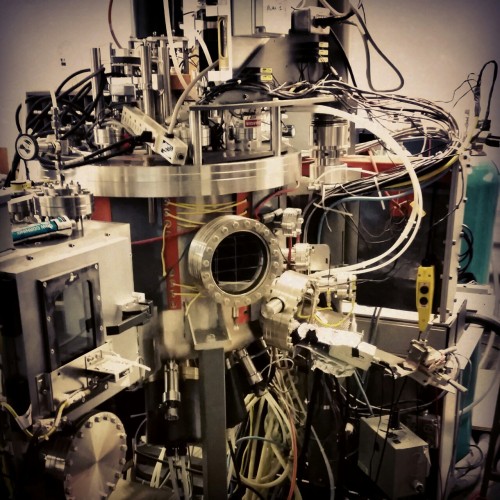
A sputter system in our lab.
Sputter deposition is a fabrication technique used to deposit thin films of a particular material onto a sample. The film can then be patterned using lithography into, for example, electrical contacts for your device. It is commonly used in the semiconductor industry to make integrated circuits.
First a gaseous plasma of ions, typically argon, is created in the sputter chamber. Ions in the plasma are then accelerated into a large piece of the material to be deposited, called the target, causing atoms to be ejected from the surface. Atoms that reach the sample or substrate are redeposited, forming a thin film over time.
Post link
Platinum plasma in our sputter system.
Plasma is the fourth state of matter (in addition to solids, liquids, and gases) that can be best described as an ionized gas. It is a high energy state, in which there is sufficient energy to strip electrons from atoms or molecules in a gas. The plasma glows because when the electrons recombine with atoms, energy is released in the form of light. The color of the light depends on the composition of the plasma. The plasma in this picture is purple mainly because of the argon ions used to bombard the platinum.
Surprisingly, even though plasma is probably one of the least known of the states of matter, it is the most common in the universe. Examples of plasmas encountered in nature include lightning, some types of flames, nebulae, and stars.
Post link