#organoids
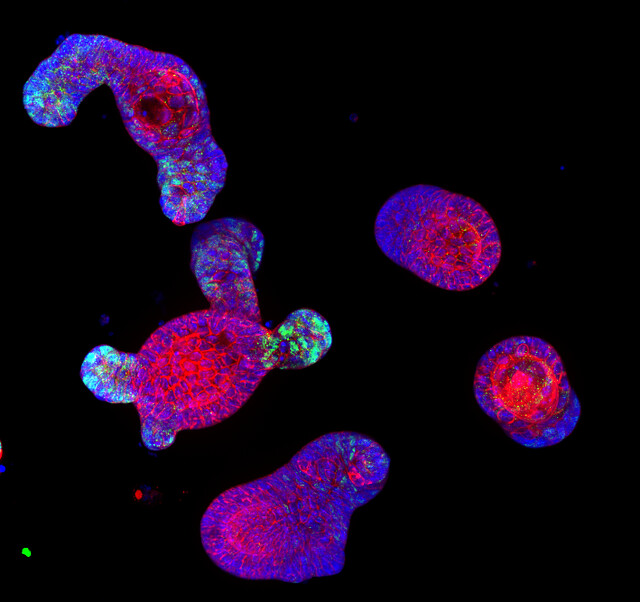
“Confocal immunofluorescence image of intestinal organoid” by Denise Serra, University of Basel by SNSF Scientific Image Competition
Via Flickr:
Entry in category 1. Object of study; © CC-BY-NC-ND: Denise Serra This image shows immuno-staining of mature intestinal organoids, complex 3-D structures that resemble both morphologically and functionally the intestinal epithelium. Intestinal organoids are great model system to study how the epithelium develops, regenerates after injury and maintain its physiological state. The image has been taken with a confocal microscope, in blue are nuclei (DAPI), in red a membrane protein and in green a transcription factor.
Gene Therapy Reverses Effects of Autism-Linked Mutation in Brain Organoids
In a study published May 02, 2022 in Nature Communications, scientists at University of California San Diego School of Medicine used lab-grown human brain organoids to learn how a genetic mutation associated with autism disrupts neural development. Recovering the function of this single gene using gene therapy tools was effective in rescuing neural structure and function.
Autism spectrum disorders (ASD) and schizophrenia have been linked to mutations in Transcription Factor 4 (TCF4), an essential gene in brain development. Transcription factors regulate when other genes are turned on or off, so their presence, or lack thereof, can have a domino effect in the developing embryo. Still, little is known about what happens to the human brain when TCF4 is mutated.
To explore this question, the research team focused on Pitt-Hopkins Syndrome, an ASD specifically caused by mutations in TCF4. Children with the genetic condition have profound cognitive and motor disabilities and are typically non-verbal.
Using stem cell technology, the researchers created brain organoids, or “mini-brains,” using cells from Pitt-Hopkins Syndrome patients, and compared their neurodevelopment to controls.
They found that fewer neurons were produced in the TCF4-mutated organoids, and these cells were less excitable than normal. They also often remained clustered together instead of arranging themselves into finely-tuned neural circuits. This atypical cellular architecture disrupted the flow of neural activity in the mutated brain organoid, which authors said would likely contribute to impaired cognitive and motor function down the line.
The team thus tested two different gene therapy strategies for recovering the functional TCF4 gene in brain tissue. Both methods effectively increased TCF4 levels, and in doing so, corrected Pitt-Hopkins Syndrome phenotypes at molecular, cellular and electrophysiological scales.
“The fact that we can correct this one gene and the entire neural system reestablishes itself, even at a functional level, is amazing,” said senior study author Alysson R. Muotri, PhD, professor at UC San Diego School of Medicine.
The team is currently optimizing their recently licensed gene therapy tools in preparation for future clinical trials, in which spinal injections of a genetic vector would hopefully recover TCF4 function in the brain.
— Nicole Mlynaryk
Post link
Brain organoids provide insight into the mechanism of a difficult-to-treat seizure disorder
Brain cells, or neurons, communicate through organized electrical bursts to control body processes like walking, talking and breathing. Sometimes, those electrical bursts can become disorganized and cause seizures, or epilepsy if the seizures are recurring. Focal cortical dysplasia — a brain disease characterized by abnormal balloon cells in the outer layer of the brain — is the leading cause of medication-resistant epilepsy. Some cases are caused by spontaneous genetic mutations, but the majority have an unknown cause. Treatment options are limited to invasive brain surgery, which may be ineffective.
In a new study, published online December 27, 2021 in Brain, an international collaboration between teams of researchers led by senior authors Alysson Muotri, PhD, director of the Stem Cell Program at the University of California San Diego School of Medicine and Iscia Lopes Cendes, PhD, professor in the Department of Translational Medicine at the University of Campinas, Brazil, describe a new laboratory model for focal cortical dysplasia using small floating balls of human brain cells called brain organoids.
Using a method called “reprogramming,” researchers are able to take skin cells from a skin biopsy and turn them into pluripotent stem cells. These stem cells can transform into any cell in the body — even tissues like brain organoids — and retain the same genetic material as the patient that received the skin biopsy, making it easier to personalize medicine.
The lead author of the study, Simoni Avancini, PhD, generated brain organoids from stem cells derived from patients with focal cortical dysplasia and compared them to brain organoids derived from healthy patients.
The researchers mimicked several aspects of the disease using the new model. They observed abnormal neurons, abnormal balloon cells, less actively dividing cells and more electrical bursts between the neurons. The results suggested that, at least in these patients, spontaneous genetic mutations do not cause focal cortical dysplasia, and it may be caused by unknown inherited mutations.
Inside brain organoids are sunflower-shaped areas called neural rosettes. where cells divide and mature into neurons. Precursor cells divide and fill the inner circle. Maturing neurons grow out of that circle like the petals of a sunflower. To investigate why brain organoids from patients with focal cortical dysplasia had less actively dividing cells, the researchers zoomed in on those neural rosettes and discovered differences in the expression of ZO-1 — a protein that helps cells stick together.
Unlike brain organoids from healthy patients where ZO-1 forms a smooth outline around the inner circle, brain organoids from patients with focal cortical dysplasia show ZO-1 as disorganized points within the inner circle. This led the researchers to investigate RHOA — a gene that regulates ZO-1 — in diseased brain organoids, and they discovered decreased expression of this gene compared to healthy brain organoids, suggesting that the decrease in actively dividing cells is caused by abnormal RHOA regulation.
Overall, these findings offer new insights into the mechanisms underlying focal cortical dysplasia, write the authors.
“We hope that this model will be useful to test and screen new theories and new ideas regarding focal cortical dysplasia, as well as finding novel treatments for this condition,” said Muotri.
— Gabriela Goldberg, graduate student

Brain organoids derived from a healthy person (left) compared to a person with focal cortical dysplasia (right).
Click to watch a video with Professor Lopes Cendes.