#stem cells
Scientists used human stem cells to build a new rat intestine
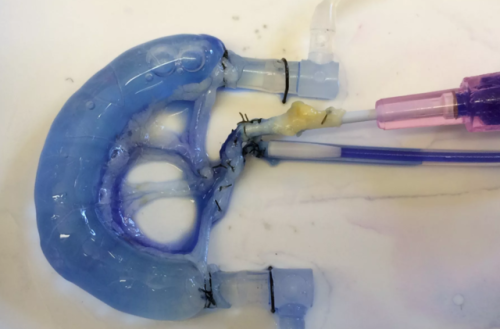
- Scientists have built a new rat intestine by combining part of the animal’s own bowel with human stem cells. One day, this method could be used in humans with intestinal problems who currently have to rely on organ transplants.
- A variety of diseases, including Crohn’s disease, lead to people having short bowels, which makes it harder for their bodies to absorb nutrients.
- One common solution is bowel transplant, but there is a shortage of intestines and, as with all transplants, the patient’s body often rejects the new organ. For a study published today in the journal Nature Communications, researchers grew new intestines in the lab. Read More
Post link
Stem cells in the skin - stem cells labelled in red are found in special microenvironments where they are surrounded by other types of cell.
Post link
DECEMBER 6 - NINA TANDON
Nina Tandon, CEO and cofounder of EpiBone, is revolutionizing medicine. Her company is the first in the world to use a patient’s stem cells to grow human bone that can then be used to repair bone defects like bone loss.
Ideally, these bones can be grown to the exact shape and size needed and are easily implanted into the body because they are made from the patient’s own cells. Tandon was named a TED senior fellow last year and she’s also one of Business Insider’s “40 under 40: People to watch in 2015.”
Text for today’s post was taken from the Business Insider piece “The 15 Most Amazing Women In Science Today”. Read even more about Nina and her company in the publication’s November 2014 profile here.
Post link
Opinion: Why science needs philosophy
By Lucy Laplane, et. al.
Despite the tight historical links between science and philosophy, present-day scientists often perceive philosophy as completely different from, and even antagonistic to, science. We argue here that, to the contrary, philosophy can have an important and productive impact on science.
Post link
Unexpected stem cell factories found inside teeth
by Sarah C. P. Williams || 30 July 2014 || Science/AAAS | News
Development is typically thought to be a one-way street. Stem cells produce cells that mature into specific types, such as the neurons and glia that compose nervous systems, but the reverse isn’t supposed to happen.
Yetresearchers have now discovered nervous system cells transforming back into stem cells in a very surprising place: inside teeth. This unexpected source of stem cells potentially offers scientists a new starting point from which to grow human tissues for therapeutic or research purposes without using embryos.
Post link
Nerve repair, with help from stem cells
A new approach to repairing peripheral nerves marries the regenerating power of gingiva-derived mesenchymal stem cells with a biological scaffold to enable the functional recovery of nerves following a facial injury, according to a study by a cross-disciplinary team from the University of Pennsylvania School of Dental Medicine and Perelman School of Medicine.
Faced with repairing a major nerve injury to the face or mouth, skilled surgeons can take a nerve from an arm or leg and use to it restore movement or sensation to the original site of trauma. This approach, known as a nerve autograft, is the standard of care for nerve repair, but has its shortcomings. Besides taking a toll on a previously uninjured body part, the procedure doesn’t always result in complete and functional nerve regrowth, especially for larger injuries.
Scientists and clinicians have recently been employing a different strategy for regrowing functional nerves involving commercially-available scaffolds to guide nerve growth. In experimental approaches, these scaffolds are infused with growth factors and cells to support regeneration. But to date, these efforts have not been completely successful. Recovery can fall short due to a failure to coax large numbers of regenerating axons to cross the graft and then adequately mature and regrow myelin, the insulating material around peripheral nerves that allows them to fire quickly and efficiently.
In an innovative approach to guided nerve repair, shared in the journal npjRegenerative Medicine, the Penn team coaxed human gingiva-derived mesenchymal stem cells (GMSCs) to grow Schwann-like cells, the pro-regenerative cells of the peripheral nervous system that make myelin and neural growth factors. The current work demonstrated that infusing a scaffold with these cells and using them to guide the repair of facial nerve injuries in an animal model had the same effectiveness as an autograft procedure.
“Instead of an autograft, which causes unnecessary morbidity, we wanted to create a biological approach and use the regenerating ability of stem cells,” says Anh Le, senior author on the study and chair and a professor in the Department of Oral and Maxillofacial Surgery/Pharmacology in Penn’s School of Dental Medicine. “To be able to recreate nerve cells in this way is really a new paradigm.”
For more than a decade, Le’s lab has pioneered the use of GMSCs to treat several inflammatory diseases and to regrow a variety of types of craniofacial tissue. Gingival tissue is easily extracted and heals rapidly, offering an accessible source of GMSCs. In fact, gingival tissue is often discarded from routine dental procedures. Le says the potential of GMSCs to help in nerve regrowth also owes in part to the cells’ common lineage. “Embryologically, we know that craniofacial tissue is derived from the same neural crest progenitor cells as nerves,” Le says. “That’s part of the beauty of this system.”
Le and colleagues led by Qunzhou Zhang, now a faculty member at Penn Dental Medicine, were able to apply their previous understanding of GMSCs to grow them in a collagen matrix using specific conditions that encouraged the cells to grow more like Schwann cells, the cells’ identity confirmed with a variety of genetic markers.
“We observed this very interesting phenomenon,” says Le, “that when we changed that matrix density and suspended the cells three-dimensionally, they changed to have more neural crest properties, like Schwann cells.”
To move the work forward, Le reached out to the Perelman School of Medicine’s D. Kacy Cullen, a bioengineer who has worked on nerve repair for 15 years. Cullen and colleagues have expertise in creating and testing nerve scaffold materials.
Using commercially available scaffolds for nerve growth, the researchers introduced the cells into collagen hydrogel. “The cells migrate into the nerve graft and create a sheet of Schwann cells,” Le says. “By doing so they are forming the functionalized nerve guidance to guide axon generation in the gap left by an injury.”
“To get host Schwann cells all throughout a bioscaffold, you’re basically approximating natural nerve repair,” Cullen says. Indeed, when Le and Cullen’s groups collaborated to implant these grafts into rodents with a facial nerve injury and then tested the results, they saw evidence of a functional repair. The animals had less facial droop than those that received an “empty” graft and nerve conduction was restored. The implanted stem cells also survived in the animals for months following the transplant.
“The animals that received nerve conduits laden with the infused cells had a performance that matched the group that received an autograft for their repair,” he says. “When you’re able to match the performance of the gold-standard procedure without a second surgery to acquire the autograft, that is definitely a technology to pursue further.”
While the current study worked at repairing a small gap in a nerve, the researchers aim to continue refining the method to try to repair larger gaps, as often arise when oral cancer necessitates surgical removal of a tumor. “The field desperately needs what have been dubbed ‘living scaffolds’ to direct the regrowth,” Cullen says.
Le notes that this approach would give patients with oral cancer or facial trauma the opportunity to use their own tissue to recover motor function and sensation and to have cosmetic improvements following a repair.
And while Le’s group focuses on the head and neck, further work on this model could translate to nerve repair in other areas of the body as well. “I’m hopeful we can continue moving this forward towards clinical application,” she says.
In a recent report, researchers at Harvard Medical School brought up a promising yet eerie prospect in stem cell research– the development of synthetic embryos. Scientists are now able to assemble stem cells that can organize into embryo-like structures, and the day may not be far off when these cells lead to the development of complex human tissues and organs that can resemble those found in adult humans.
Sheefs, which stands for “synthetic human entities with embryo-like features”, exist even today but only as simple assemblies of cells.
From The New York Times’ article:
But in the future, they may develop into far more complex forms, the researchers said, such as a beating human heart connected to a rudimentary brain, all created from stem cells. Such a Sheef might reveal important clues about how nerves control heartbeats. Scientists might be able to use other Sheefs to test out drugs for diseases such as cancer or diabetes.
However, the full potential of this avenue of research may soon push the boundaries of what is currently legally acceptable. A rule that has been in existence since 1979 prohibits lab-raised embryos from being allowed to grow for more than 14 days. Scientists have largely found this to be a reasonable restriction because of how easy it has been to follow—until recently, scientists couldn’t even come close to keeping human embryos alive for even 1 week. But this may soon change.
Last year, two teams of scientists determined how to grow human embryos for 13 days. Those advances hinted that it might be possible to allow scientists to tack on a few days more, by changing the 14-day rule to, say, a 20-day rule.
To read more about this complex new route of research, read “A New Form of Stem-Cell Engineering Raises Ethical Questions” in The New York Times.
Post link
Bone Marrow Cells (BMCs)
Whole bone marrow cells (BMCs) and bone marrow mononuclear cells (BMMCs) are the most accessible and studied source of stem cells.
- BMMCs are isolated from whole bone marrow, and contain a diverse cell population, including mesenchymal stem cells and hematopoietic progenitor cells.
Mesenchymal Stem Cells (MSCs)
MSCs can be isolated from a variety of tissues such as bone marrow, adipose, and umbilical cord; although it is not clear whether their properties are uniform (Selem, Hatzistergos and Hare, 2011).
MSCs are of particular note due to their immunoprivelegednature – a reduced expression of MHC class-I molecule, and lack of MHC class-II and co-stimulatory molecules, means they could potentially be used for allogeneic grafts (Zimmet et al., 2005). This means that they don’t produce an immune response and could be used in transplants - the body won’t reject them.
- MSCs inhibit the activity of various immune cells, including T cells, B cells, natural killer cells, and dendritic cells via cell to cell contacts and soluble factors (Laflamme and Murry, 2005).
Foetal and Umbilical Cord Cells
Embryonic stem cells (ESCs), the prototypical stem cell, can develop into all cell types in the body. However, the practical application of human ESCs remains limited due to ethical problems, teratoma formation (cancer), and immune rejection. With rapidly expanding knowledge of molecular and genetic pathways for ESC differentiation, it may become possible to avoid contamination with undifferentiated ESCs, thereby inhibiting teratogenesis when transplanted into the body (Kucia et al., 2006).
- Foetal-derived stem cells can also be isolated from the amniotic fluid, which include both pluripotent and committed stem cells.
- Umbilical cord cells can be gathered at birth and stored, eg if for treatment later on if a defect is detected in utero.
Induced pluripotent stem cells (iPSCs)
Induced pluripotent stem cells are a more attractive alternative to ESCs, as they are autologous. This means cells can be taken from an individual, ‘reset’ back to their stem cell stage, and then administered to that same individual to avoid rejection. Pluripotency transcription factors are introduced to adult terminally differentiated somatic cells, such as dermal fibroblasts, in a novel strategy which ‘reprograms’ the cells back to an embryonic stem cell-like stage (Yu et al., 2007).
Despite slight epigenetic differences associated with reprogramming, iPSCs fully resemble ESCs in terms of differentiation capacity, morphology and gene expression profile; and have the ability to differentiate into other cells. Ethical and immune response dilemmas are bypassed by the autologous nature of iPSCs, however clinical application is not yet on the horizon due to their teratogenic potential and the oncogenes and virus vectors required for the current method of pluripotent induction (Yamanaka and Takahashi, 2006).
Skeletal myoblasts (SM)
Skeletal myoblasts (satellite cells) are derived from skeletal muscle and have the capacity to differentiate into muscle fibre, which makes them obvious candidates for treating conditions such as heart damage following infarction. However, clinical trials have been halted as SM have been observed to couple with resident cardiomyocytes, resulting in dysfunctional electrocardiology and arrhythmias, and have struggled to transdifferentiate into cardiomyocytes in vivo (Reinecke, Poppa and Murry, 2002).
Stem cells are cells in the body that don’t yet have any role (undifferentiated or partially differentiated), and can change to become almost any cell type.
- Canproliferate (divide to make more cells) indefinitelyto make more of the same stem cells.
- They are the earliest type of cell in a cell lineage (if a cell was an adult human, stem cells would be the foetus)
- found in both embryonic and adult organisms, but they have slightly different properties in each
- Can also be made in a lab by reprogramming other cells - resetting them back to stem cell stage.

Inembryonic development (a baby forming in the womb), pluripotent stem cells develop at the blastocyst stage (3-4 days) and differentiate into all the cells of the human body as the foetus develops.
Stem cells do exist in the adult body, however they are not pluripotent - they are unipotent or multipotent - this means they can only differentiate into one or a few cell types respectively.
Adult stem cells
Adult stem cells are found in a few select locations in the body, known as niches, such as:
- the brain
- bone marrow
- blood and blood vessels
- skeletal muscles
- liver
- gonads
They exist to replenish rapidly lost cell types and include hematopoieticstem cells, which replenish blood and immune cells,basal cells, which maintain the skin epithelium, and mesenchymal stem cells, which maintain bone, cartilage, muscle and fat cells. Adult stem cells are a small minorityof cells.
Stem cell bandages
Bandages and band aids containing stem cells to help heal wounds are closer to reality after scientists found a way to keep stem cells alive at room temperature.
By encasing stem cells in a frogspawn-like gel made from a seaweed extract called alginate, scientists at Newcastle University can put them inside bandages, which could be used to help speed up healing from ulcers or burns for example.
The gel retains the cells so that they don’t leave the bandage – it’s the chemicals these cells make that actually do the healing.
The low-cost discovery also has lots of other potential applications, because until now stem cells have had to be kept in specialised conditions; at 37°C, in atmospheric oxygen and 5% carbon dioxide.
Alginate is a natural material extracted from seaweed that is used in cosmetics and food manufacturing and is already used in wound dressings, without stem cells, to keep burns moist.
Images: Newcastle University
Post link
Gene Therapy Reverses Effects of Autism-Linked Mutation in Brain Organoids
In a study published May 02, 2022 in Nature Communications, scientists at University of California San Diego School of Medicine used lab-grown human brain organoids to learn how a genetic mutation associated with autism disrupts neural development. Recovering the function of this single gene using gene therapy tools was effective in rescuing neural structure and function.
Autism spectrum disorders (ASD) and schizophrenia have been linked to mutations in Transcription Factor 4 (TCF4), an essential gene in brain development. Transcription factors regulate when other genes are turned on or off, so their presence, or lack thereof, can have a domino effect in the developing embryo. Still, little is known about what happens to the human brain when TCF4 is mutated.
To explore this question, the research team focused on Pitt-Hopkins Syndrome, an ASD specifically caused by mutations in TCF4. Children with the genetic condition have profound cognitive and motor disabilities and are typically non-verbal.
Using stem cell technology, the researchers created brain organoids, or “mini-brains,” using cells from Pitt-Hopkins Syndrome patients, and compared their neurodevelopment to controls.
They found that fewer neurons were produced in the TCF4-mutated organoids, and these cells were less excitable than normal. They also often remained clustered together instead of arranging themselves into finely-tuned neural circuits. This atypical cellular architecture disrupted the flow of neural activity in the mutated brain organoid, which authors said would likely contribute to impaired cognitive and motor function down the line.
The team thus tested two different gene therapy strategies for recovering the functional TCF4 gene in brain tissue. Both methods effectively increased TCF4 levels, and in doing so, corrected Pitt-Hopkins Syndrome phenotypes at molecular, cellular and electrophysiological scales.
“The fact that we can correct this one gene and the entire neural system reestablishes itself, even at a functional level, is amazing,” said senior study author Alysson R. Muotri, PhD, professor at UC San Diego School of Medicine.
The team is currently optimizing their recently licensed gene therapy tools in preparation for future clinical trials, in which spinal injections of a genetic vector would hopefully recover TCF4 function in the brain.
— Nicole Mlynaryk
Post link
Brain organoids provide insight into the mechanism of a difficult-to-treat seizure disorder
Brain cells, or neurons, communicate through organized electrical bursts to control body processes like walking, talking and breathing. Sometimes, those electrical bursts can become disorganized and cause seizures, or epilepsy if the seizures are recurring. Focal cortical dysplasia — a brain disease characterized by abnormal balloon cells in the outer layer of the brain — is the leading cause of medication-resistant epilepsy. Some cases are caused by spontaneous genetic mutations, but the majority have an unknown cause. Treatment options are limited to invasive brain surgery, which may be ineffective.
In a new study, published online December 27, 2021 in Brain, an international collaboration between teams of researchers led by senior authors Alysson Muotri, PhD, director of the Stem Cell Program at the University of California San Diego School of Medicine and Iscia Lopes Cendes, PhD, professor in the Department of Translational Medicine at the University of Campinas, Brazil, describe a new laboratory model for focal cortical dysplasia using small floating balls of human brain cells called brain organoids.
Using a method called “reprogramming,” researchers are able to take skin cells from a skin biopsy and turn them into pluripotent stem cells. These stem cells can transform into any cell in the body — even tissues like brain organoids — and retain the same genetic material as the patient that received the skin biopsy, making it easier to personalize medicine.
The lead author of the study, Simoni Avancini, PhD, generated brain organoids from stem cells derived from patients with focal cortical dysplasia and compared them to brain organoids derived from healthy patients.
The researchers mimicked several aspects of the disease using the new model. They observed abnormal neurons, abnormal balloon cells, less actively dividing cells and more electrical bursts between the neurons. The results suggested that, at least in these patients, spontaneous genetic mutations do not cause focal cortical dysplasia, and it may be caused by unknown inherited mutations.
Inside brain organoids are sunflower-shaped areas called neural rosettes. where cells divide and mature into neurons. Precursor cells divide and fill the inner circle. Maturing neurons grow out of that circle like the petals of a sunflower. To investigate why brain organoids from patients with focal cortical dysplasia had less actively dividing cells, the researchers zoomed in on those neural rosettes and discovered differences in the expression of ZO-1 — a protein that helps cells stick together.
Unlike brain organoids from healthy patients where ZO-1 forms a smooth outline around the inner circle, brain organoids from patients with focal cortical dysplasia show ZO-1 as disorganized points within the inner circle. This led the researchers to investigate RHOA — a gene that regulates ZO-1 — in diseased brain organoids, and they discovered decreased expression of this gene compared to healthy brain organoids, suggesting that the decrease in actively dividing cells is caused by abnormal RHOA regulation.
Overall, these findings offer new insights into the mechanisms underlying focal cortical dysplasia, write the authors.
“We hope that this model will be useful to test and screen new theories and new ideas regarding focal cortical dysplasia, as well as finding novel treatments for this condition,” said Muotri.
— Gabriela Goldberg, graduate student

Brain organoids derived from a healthy person (left) compared to a person with focal cortical dysplasia (right).
Click to watch a video with Professor Lopes Cendes.