#molecular biology
Tome Sweet Tome
The surfaces of all cells in nature are festooned with a complex and diverse array of sugar chains (called glycans). These perform a wide variety of biological functions, from the proper folding of proteins to cell-to-cell interactions. Their ubiquity in nature underscores their essentialness to complex life.
This week, the fourth edition of “Essentials of Glycobiology” (the study of glycans) was published by Cold Spring Harbor Laboratory Press. It’s a continuation and updating of landmark work by a consortium of editors, led by Ajit Varki, MD, Distinguished Professor in the departments of Medicine and Cellular and Molecular Medicine at UC San Diego School of Medicine, with contributions from a number of UC San Diego scientists and physicians, including Jeffrey D. Esko, PhD, Distinguished Professor of cellular and molecular medicine; Pascal Gagneux, PhD, professor of pathology and anthropology, and Kamil Godula, PhD, associate professor of chemistry and biochemistry, and Amanda Lewis PhD, professor of obstetrics-gynecology and reproductive science.
Varki and Esko are also founding directors of the Glycobiology Research and Training Center (GRTC) at UC San Diego, established in 1999, and have recently handed over leadership to Lewis and Godula.
Glycobiology is a relatively new scientific discipline. The term was only coined in 1988, recognizing the combining of carbohydrate chemistry and biochemistry to focus on glycans, which have since proven to have a multitude of diverse and often critical roles in biology.
They have been linked to human origins and as a key evolutionary marker. They are found to both inhibit and promote tumor growth; and the presence of a particular sialic acid in red meat may be linked to increased cancer risk in humans. Another class of glycans called glycosaminoglycans have been shown by Esko and colleagues to be involved in COVID-19 coronavirus pathogenesis. The cover of the fourth edition presents an all-atom model of infamous spike protein of the pandemic virus, emphasizing the massive array of glycan chains modelled by UC San Diego professor of biology Rommie Amaro.
Varki, Esko and colleagues at the GRTC have been central to many of the advances in glycobiology, and the textbook, which originally debuted in 1999, has been an enduring effort to broadly introduce and describe the rapidly changing discipline.
For example, the second edition of “Essentials of Glycobiology,” published in 2008, appeared simultaneously in print from the Cold Spring Harbor Laboratory Press, and free online to reach a wider audience. Subsequent editions have also been free online at the National Center for Biotechnology Information at the National Library of Medicine.
“This approach ensures that everyone, from the layperson to the high school student to the graduate student in a developing country, has free access to the knowledge the book contains, while increasing awareness of the availability of a printed edition that may be more suitable for some readers’ requirements,” said Varki at the time.
— Scott LaFee
Pictured above: In this electron micrograph, the surface of a bacterium is fuzzy with a coating of glycans.
Post link
Gene Therapy Reverses Effects of Autism-Linked Mutation in Brain Organoids
In a study published May 02, 2022 in Nature Communications, scientists at University of California San Diego School of Medicine used lab-grown human brain organoids to learn how a genetic mutation associated with autism disrupts neural development. Recovering the function of this single gene using gene therapy tools was effective in rescuing neural structure and function.
Autism spectrum disorders (ASD) and schizophrenia have been linked to mutations in Transcription Factor 4 (TCF4), an essential gene in brain development. Transcription factors regulate when other genes are turned on or off, so their presence, or lack thereof, can have a domino effect in the developing embryo. Still, little is known about what happens to the human brain when TCF4 is mutated.
To explore this question, the research team focused on Pitt-Hopkins Syndrome, an ASD specifically caused by mutations in TCF4. Children with the genetic condition have profound cognitive and motor disabilities and are typically non-verbal.
Using stem cell technology, the researchers created brain organoids, or “mini-brains,” using cells from Pitt-Hopkins Syndrome patients, and compared their neurodevelopment to controls.
They found that fewer neurons were produced in the TCF4-mutated organoids, and these cells were less excitable than normal. They also often remained clustered together instead of arranging themselves into finely-tuned neural circuits. This atypical cellular architecture disrupted the flow of neural activity in the mutated brain organoid, which authors said would likely contribute to impaired cognitive and motor function down the line.
The team thus tested two different gene therapy strategies for recovering the functional TCF4 gene in brain tissue. Both methods effectively increased TCF4 levels, and in doing so, corrected Pitt-Hopkins Syndrome phenotypes at molecular, cellular and electrophysiological scales.
“The fact that we can correct this one gene and the entire neural system reestablishes itself, even at a functional level, is amazing,” said senior study author Alysson R. Muotri, PhD, professor at UC San Diego School of Medicine.
The team is currently optimizing their recently licensed gene therapy tools in preparation for future clinical trials, in which spinal injections of a genetic vector would hopefully recover TCF4 function in the brain.
— Nicole Mlynaryk
Post link
Excess Neuropeptides Disrupt Lung Function in Infant Disease and COVID-19
Excess fluid in the lung can significantly disrupt lung function and gas exchange, but researchers at University of California San Diego School of Medicine were surprised to find that neuropeptides may be to blame.
In a study published March 17, 2022 in the journal Developmental Cell, scientists show that excessive neuropeptide secretion by neuroendocrine cells in the lungs can lead to fluid buildup and poor oxygenation. However, blocking the neuropeptide signals with receptor antagonists prevented the leakage and improved blood-oxygen levels, suggesting that neuropeptides may be a promising therapeutic target for conditions marked by excess lung fluid.
This mechanism was discovered in the context of neuroendocrine cell hyperplasia of infancy (NEHI), a lung disease affecting infants in which lung size and structure appear normal but blood-oxygen levels are consistently low. Its defining feature is an increase in the number of pulmonary neuroendocrine cells (PNECs), but until now, physicians did not know how these cells contributed to the disease.
In the new study, researchers confirmed that PNECs and their neuropeptide products are the drivers of NEHI, but also showed that PNEC numbers were increased in the lungs of COVID-19 patients with excess lung fluid. This suggests a similar mechanism may contribute to COVID-19 symptoms.
The study was led by Xin Sun, PhD, professor of pediatrics at UC San Diego School of Medicine and the Division of Biological Sciences.
“We were surprised to find that neuropeptides can play such a major role in gas exchange,” said Sun. “Researchers are just starting to appreciate the relationship between the nervous system and the lungs, but the more we understand it, the more we can modulate it to treat disease.”
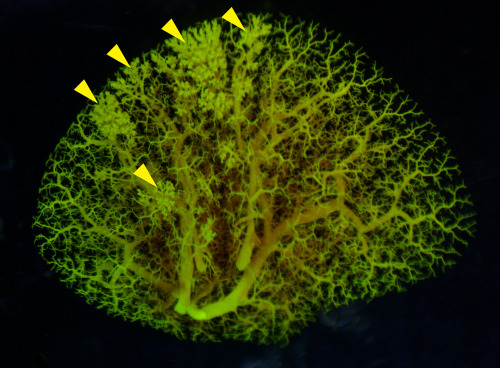
Pictured above: An angiogram of blood vessels in the NEHI mouse lung shows multiple sites of fluid leakage, marked by yellow arrowheads.
— Nicole Mlynaryk, Bigelow Science Communication Fellow
Post link