#academic medicine
In recent years, hallucinogens ranging from LSD and ecstasy (MDMA/Molly) to salvia divinorum and ketamine have garnered renewed interest as potential as therapeutics for a variety of psychiatric conditions. Both LSD and ketamine, for example, are being widely studied as a treatment for major depression.
In a study published online April 28, 2022 in the journal Addictive Behaviors, researchers at UC San Diego School of Medicine and New York University investigated how use of these substances outside of medical settings relates to subsequent psychological distress, depression and suicidality.
They examined data from a representative sampling of noninstitutionalized adults (2015-2020) who had reported specific drug use on the National Survey on Drug Use and Health, and whether that use was associated with any reported serious psychological distress, major depressive episode (MDE) or suicidality.
The researchers found that LSD was associated with an increased likelihood of MDE and suicidal thinking. Salvia divinorum, a plant species with psychoactive properties when its leaves are consumed by chewing, smoking or as a tea, was linked to increased suicidal thinking. The hallucinogens DMT, AMT and Foxy were associated with suicidal planning.

Sometimes called “Maria Pastora” or “Sally-D,” Salvia divinorum contains opioid-like compounds that induce hallucinations when the leaves are chewed, smoke or brewed in a tea. Researcher found the plant also induces an increased likelihood of suicidal thinking.
Conversely, ecstasy use was associated with a decreased likelihood of serious psychological distress, MDE and suicidal planning.
“The findings suggest there are differences among specific hallucinogens with respect to depression and suicidality,” wrote authors Kevin H. Yang, a fourth year medical student; Benjamin H. Han, MD, an assistant adjunct professor at UC San Diego School of Medicine; and Joseph J. Palamar of New York University. “More research is warranted to understand consequences of and risk factors for hallucinogen use outside of medical settings among adults experiencing depression or suicidality.”
— Scott LaFee
If you or someone you know may be considering suicide, contact the National Suicide Prevention Lifeline at 1-800-273-8255 (En Español: 1-888-628-9454; Deaf and Hard of Hearing: 1-800-799-4889) or the Crisis Text Line by texting HOME to 741741.
Gene Therapy Reverses Effects of Autism-Linked Mutation in Brain Organoids
In a study published May 02, 2022 in Nature Communications, scientists at University of California San Diego School of Medicine used lab-grown human brain organoids to learn how a genetic mutation associated with autism disrupts neural development. Recovering the function of this single gene using gene therapy tools was effective in rescuing neural structure and function.
Autism spectrum disorders (ASD) and schizophrenia have been linked to mutations in Transcription Factor 4 (TCF4), an essential gene in brain development. Transcription factors regulate when other genes are turned on or off, so their presence, or lack thereof, can have a domino effect in the developing embryo. Still, little is known about what happens to the human brain when TCF4 is mutated.
To explore this question, the research team focused on Pitt-Hopkins Syndrome, an ASD specifically caused by mutations in TCF4. Children with the genetic condition have profound cognitive and motor disabilities and are typically non-verbal.
Using stem cell technology, the researchers created brain organoids, or “mini-brains,” using cells from Pitt-Hopkins Syndrome patients, and compared their neurodevelopment to controls.
They found that fewer neurons were produced in the TCF4-mutated organoids, and these cells were less excitable than normal. They also often remained clustered together instead of arranging themselves into finely-tuned neural circuits. This atypical cellular architecture disrupted the flow of neural activity in the mutated brain organoid, which authors said would likely contribute to impaired cognitive and motor function down the line.
The team thus tested two different gene therapy strategies for recovering the functional TCF4 gene in brain tissue. Both methods effectively increased TCF4 levels, and in doing so, corrected Pitt-Hopkins Syndrome phenotypes at molecular, cellular and electrophysiological scales.
“The fact that we can correct this one gene and the entire neural system reestablishes itself, even at a functional level, is amazing,” said senior study author Alysson R. Muotri, PhD, professor at UC San Diego School of Medicine.
The team is currently optimizing their recently licensed gene therapy tools in preparation for future clinical trials, in which spinal injections of a genetic vector would hopefully recover TCF4 function in the brain.
— Nicole Mlynaryk
Post link
Harms of Vaping Hang in the Air
With the increasing popularity of e-cigarettes or vaping, there has been a concurrent rise in “e-cigarette or vaping product use-associate lung injury,” dubbed EVALI. In 2019, according to published data, more than half of patients diagnosed with EVALI in the United States required hospitalization.
In a paper published March 30, 2022 in the journal CHEST, a multi-institution team of researchers, including Laura E. Crotty Alexander, MD, associate professor of medicine in the UC San Diego School of Medicine and a pulmonary specialist, outline best health care practices for treating EVALI patients.
“Not long ago, there was tremendous interest in vaping-related lung injuries. But I think, especially in light of the COVID-19 pandemic, many people believe this problem has gone away,” said first author Don Hays, MD, a pulmonologist at Cincinnati Children’s Hospital.
“The truth is that it hasn’t. These injuries are still being seen, though we’re not positive on the frequency because the CDC has ceased collecting data since the pandemic began. The goal of this study is twofold: to provide information and guidance on treating EVALI patients, and also to put forth a reminder that this is still a problem.”
EVALI is characterized by respiratory symptoms, such as cough, shortness of breath and chest pain, combined with gastrointestinal symptoms, such as nausea, vomiting and diarrhea. The new study reviewed CDC data regarding 2,708 confirmed or probable EVALI patients requiring hospital admission between August 2019 and January 2020. The study reported that 93 percent of the patients survived to discharge, but 88.5 percent required respiratory support.
Given that EVALI symptoms can be similar to common respiratory infections, such as influenza and COVID-19, the authors said it is important to determine whether a patient has a history of e-cigarette use, particularly within the last three months.
Alexander said the EVALI epidemic in 2019 was primarily due to the addition on Vitamin E acetate to e-cigarettes already containing THC, the psychoactive compound in marijuana, but also noted that e-cigarettes containing nicotine were linked to EVALI before and after 2019.
“We need to continue to warn both THC and nicotine e-cigarette vapers about the potential for acute lung injury,” said Alexander, who noted that UC San Diego Health averages two EVALI hospitalizations monthly.
“In health care, it is critical to be aware of, and understand, what’s going on in the public domain, in order to be suspicious of what might be happening with an individual patient. This is why public health is so important,” said Hayes. “The fact is, the average doctor may only see one or two EVALI cases, so by utilizing this panel of experts, who see EVALI cases more frequently, we’re able to provide guidance to questions like, ‘What should we be doing, how do we manage this, and should we be doing certain types of diagnostic tests?’”
Alexander agreed: “As clinicians become more aware of the health effects of e-cigarettes, we are hopeful that more accurate inhalation histories will be taken and documented, allowing us to accurately quantify e-cigarette driven diseases and outcomes.”
— Scott LaFee
Post link
Excess Neuropeptides Disrupt Lung Function in Infant Disease and COVID-19
Excess fluid in the lung can significantly disrupt lung function and gas exchange, but researchers at University of California San Diego School of Medicine were surprised to find that neuropeptides may be to blame.
In a study published March 17, 2022 in the journal Developmental Cell, scientists show that excessive neuropeptide secretion by neuroendocrine cells in the lungs can lead to fluid buildup and poor oxygenation. However, blocking the neuropeptide signals with receptor antagonists prevented the leakage and improved blood-oxygen levels, suggesting that neuropeptides may be a promising therapeutic target for conditions marked by excess lung fluid.
This mechanism was discovered in the context of neuroendocrine cell hyperplasia of infancy (NEHI), a lung disease affecting infants in which lung size and structure appear normal but blood-oxygen levels are consistently low. Its defining feature is an increase in the number of pulmonary neuroendocrine cells (PNECs), but until now, physicians did not know how these cells contributed to the disease.
In the new study, researchers confirmed that PNECs and their neuropeptide products are the drivers of NEHI, but also showed that PNEC numbers were increased in the lungs of COVID-19 patients with excess lung fluid. This suggests a similar mechanism may contribute to COVID-19 symptoms.
The study was led by Xin Sun, PhD, professor of pediatrics at UC San Diego School of Medicine and the Division of Biological Sciences.
“We were surprised to find that neuropeptides can play such a major role in gas exchange,” said Sun. “Researchers are just starting to appreciate the relationship between the nervous system and the lungs, but the more we understand it, the more we can modulate it to treat disease.”
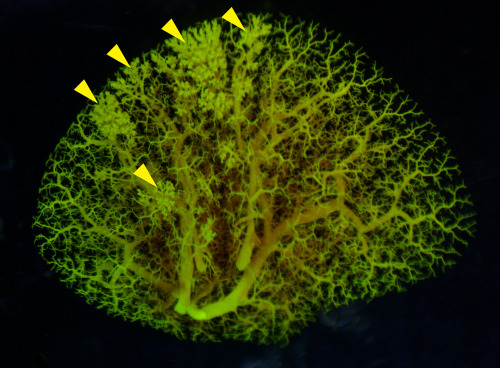
Pictured above: An angiogram of blood vessels in the NEHI mouse lung shows multiple sites of fluid leakage, marked by yellow arrowheads.
— Nicole Mlynaryk, Bigelow Science Communication Fellow
Post link
All of UsAdvances
Officially launched in 2018, the All of Us Research Program represents a massive, long-term effort to gather information from 1 million or more persons living in the United States, then use that data to accelerate health research and medical therapies. The biggest emphasis is upon gathering information on racial, ethnic and cultural groups who have historically been underrepresented or ignored in medical research.
Today, the sponsoring National Institutes of Health announced the release of the first genomic dataset generated by All of Us: nearly 100,000 whole genome sequences encompassing diverse individuals that can be used as a national resource for studies covering a wide variety of health conditions.
UC San Diego is part of the All of Us program, led by Lucila Ohno-Machado, MD, PhD, Distinguished Professor of Medicine, chair of the Department of Biomedical Informatics at UC San Diego Health, and associate dean for informatics and technology.
“As modern medicine seeks to become more precise and personalized, it necessarily requires more and more data to both understand the big picture of health and disease and, more specifically, how each person fits into the whole,” said Ohno-Machado. “With this first public genomic dataset, All of Us begins to meet its goals and expectations, allowing physicians and scientists to parse the mysteries and challenges of diseases across the health spectrum in new, individualized ways.”
— Scott LaFee
Post link
Sequencing Celebrity Mice: New Study Compares Genetics of 14 Popular Mouse Models
In a new study published on March 9, 2022 in Cell Genomics, researchers at the University of California San Diego School of Medicine present a genome-wide map comparing the genetic makeup of 14 common strains of laboratory mice.
In the century since the C57BL mouse strain was first generated, it has become the most popular laboratory rodent for biomedical research. It functions as a sort of “default” mouse, and its genetic makeup is commonly used as a “neutral” backdrop for genetic modifications that model human diseases. The specific C57BL/6J strain from The Jackson Laboratory is currently the most commonly used inbred mouse, with a closely-related C57BL/10 strain widely used in fields, such as immunology. Many additional sub-strains have since been derived from both.
Given the prevalence of these mouse strains in biology research, a comprehensive understanding of their genetic similarities and differences is valuable to researchers, but until recently, such a resource did not exist.
A team led by Abraham Palmer, PhD, and Jonathan Sebat, PhD, professors at UC San Diego School of Medicine, has now identified 352,631 single nucleotide polymorphisms (SNPs), 109,096 small insertions and deletions (INDELs), 150,344 short tandem repeats (STRs), 3,425 structural variations (SVs) and 2,826 differentially expressed genes (DEGenes) among the different strains. Most of the SNPs were clustered into 28 short segments in the genome, indicating that these genetic differences are likely due to an early introgression of an unrelated mouse, rather than recent independent mutations.
The authors say these results can now be used to guide both forward genetic approaches (wherein scientists identify a phenotypic difference between mice and look for the genetic variation that caused it) and reverse approaches (wherein scientists first identify a genetic difference and then assess whether it produces a different phenotype). Either way, they urge researchers to be aware of the unique genetic profile of their strain of choice.
— Nicole Mlynaryk, Bigelow Science Communication Fellow
Post link
When Viruses Become More Virulent
The evolution of virulence depends on the biology and interplay of infection and transmission, writes Joel O. Wertheim, PhD, associate professor in the Division of Infectious Diseases and Global Public Health at UC San Diego School of Medicine, in the journal Science.
Virulence is the degree to which a pathogen weakens, sickens or kills its host. More virulent pathogens may be less transmissible because in killing its host, it reduces the opportunity for transmission. But both virulence and transmissibility are forever linked: To maintain or increase infectiousness, a pathogen must be virulent.
In his Science article, Wertheim describes the emergence of a more virulent and transmissible variant of HIV that has spread to 102 known cases, mostly in the Netherlands. These findings, he says, have relevance to SARS-CoV-2 and the COVID-19 pandemic. It’s possible that the coronavirus is evolving toward a more benign, highly transmissible infection, similar to common cold viruses, but that outcome is not guaranteed.
SARS-CoV-2 has displayed an extraordinary ability to rapidly alter its transmissibility and virulence, and how those two factors interact over the coming months will dictate whether SARS-CoV-2 will benignly fade away or continue to be an evolving, global public health threat.
– Scott LaFee
Post link
Brain organoids provide insight into the mechanism of a difficult-to-treat seizure disorder
Brain cells, or neurons, communicate through organized electrical bursts to control body processes like walking, talking and breathing. Sometimes, those electrical bursts can become disorganized and cause seizures, or epilepsy if the seizures are recurring. Focal cortical dysplasia — a brain disease characterized by abnormal balloon cells in the outer layer of the brain — is the leading cause of medication-resistant epilepsy. Some cases are caused by spontaneous genetic mutations, but the majority have an unknown cause. Treatment options are limited to invasive brain surgery, which may be ineffective.
In a new study, published online December 27, 2021 in Brain, an international collaboration between teams of researchers led by senior authors Alysson Muotri, PhD, director of the Stem Cell Program at the University of California San Diego School of Medicine and Iscia Lopes Cendes, PhD, professor in the Department of Translational Medicine at the University of Campinas, Brazil, describe a new laboratory model for focal cortical dysplasia using small floating balls of human brain cells called brain organoids.
Using a method called “reprogramming,” researchers are able to take skin cells from a skin biopsy and turn them into pluripotent stem cells. These stem cells can transform into any cell in the body — even tissues like brain organoids — and retain the same genetic material as the patient that received the skin biopsy, making it easier to personalize medicine.
The lead author of the study, Simoni Avancini, PhD, generated brain organoids from stem cells derived from patients with focal cortical dysplasia and compared them to brain organoids derived from healthy patients.
The researchers mimicked several aspects of the disease using the new model. They observed abnormal neurons, abnormal balloon cells, less actively dividing cells and more electrical bursts between the neurons. The results suggested that, at least in these patients, spontaneous genetic mutations do not cause focal cortical dysplasia, and it may be caused by unknown inherited mutations.
Inside brain organoids are sunflower-shaped areas called neural rosettes. where cells divide and mature into neurons. Precursor cells divide and fill the inner circle. Maturing neurons grow out of that circle like the petals of a sunflower. To investigate why brain organoids from patients with focal cortical dysplasia had less actively dividing cells, the researchers zoomed in on those neural rosettes and discovered differences in the expression of ZO-1 — a protein that helps cells stick together.
Unlike brain organoids from healthy patients where ZO-1 forms a smooth outline around the inner circle, brain organoids from patients with focal cortical dysplasia show ZO-1 as disorganized points within the inner circle. This led the researchers to investigate RHOA — a gene that regulates ZO-1 — in diseased brain organoids, and they discovered decreased expression of this gene compared to healthy brain organoids, suggesting that the decrease in actively dividing cells is caused by abnormal RHOA regulation.
Overall, these findings offer new insights into the mechanisms underlying focal cortical dysplasia, write the authors.
“We hope that this model will be useful to test and screen new theories and new ideas regarding focal cortical dysplasia, as well as finding novel treatments for this condition,” said Muotri.
— Gabriela Goldberg, graduate student

Brain organoids derived from a healthy person (left) compared to a person with focal cortical dysplasia (right).
Click to watch a video with Professor Lopes Cendes.