#materials science
3-D printing hierarchical liquid-crystal-polymer structures
Biological materials from bonetospider-silk and wood are lightweight fibre composites arranged in a complex hierarchical structure, formed by directed self-assembly to demonstrate outstanding mechanical properties. When such bioinspired stiff and lightweight materials are typically developed for applications in aircraft, automobiles and biomedical implants, their manufacture requires energy and labor-intensive fabrication processes. The manufactured materials also exhibit brittle fracture characteristics with difficulty to shapeandrecycle, in stark contrast to the mechanical properties of nature. Existing polymer-based lightweight structure fabrication is limited to 3-D printing, with poor mechanical strength and orientation, while highly oriented stiff polymers are restricted to construct simple geometries. In an effort to combine the freedom of structural shaping with molecular orientation, 3-D printing of liquid-crystal polymers was recently exploited. Although desirable shape-morphing effects were attained, the Young’s modulus of the soft elastomers were lower than high-performance liquid-crystal synthetic fibers due to their molecular structure.
To fully exploit the shaping freedom of 3-D printing and favorable mechanical properties of molecularly oriented liquid-crystal polymers (LCP), a team of scientists at the Department of Materials, ETH Zürich, proposed a novel approach. The strategy followed two design principles that are used in nature to form tough biological materials. Initially, anisotropy was achieved in the printing process via self-assembly of the LCP ink along the print path. Thereafter, complex-shaping capacity offered by the 3-D printing process was exploited to tailor the local stiffness and strength of the structure based on environmental loading conditions. In the study, Silvan Gantenbein and co-workers demonstrated an approach to generate 3-D lightweight, recyclable structures with hierarchical architecture and complex geometries for unprecedented stiffness and toughness. The results are now published in Nature.
Post link
Let there be light
The vials shown here contain a molecule that researchers can activate with light to affect biological processes in mammals. It’s part of a class of potential smart drugs that are under development to treat ailments including diabetes, blindness, and the side effects of chemotherapy. Because the molecules turn on only when hit with a certain wavelength of light, researchers can control when and where the compounds are active in the body. This photo was taken by Dusan Kolarski, a graduate student at the University of Groningen in the lab of Ben Feringa, who won the 2016 Nobel Prize in Chemistry for his work on molecular machines. Feringa’s group is working to create antibiotics that can be activated with light and thus may pose a lower risk of bacterial resistance. The team also wants to make water-soluble motors for applications in photopharmacology.—ALEXANDRA TAYLOR
Submitted by Dusan Kolarski
Do science. Take pictures. Win money. Enter our photo contest here.
Related C&EN Content:
Photoswitchable drugs could light the way to more targeted treatments
Flipping A Light Switch For Antibiotics
Light-activated gel releases insulin for potential diabetes treatment
Post link
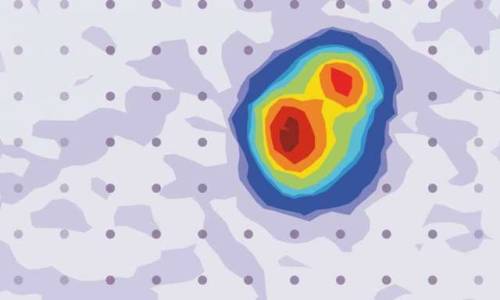
A mysterious insulating phenomenon in a superconductor
Leiden physicist Milan Allan and his group have discovered an apparent paradox within a material that has zero electrical resistance. They report trapped charges, although charges should, in theory, keep flowing in the absence of resistance. The discovery could provide a missing piece of one of the big puzzles in physics today—high-temperature superconductivity. The results are published in Nature Physics.
A material can either be insulating or conductive. In an insulator, an extra electron will get trapped. Thus, no electric current flows in insulators. In a conductor, extra electrons will immediately flow. The more conductive the material is, the faster the electrons will flow.
The research group of Leiden physicist Milan Allan was therefore surprised to discover charge trapping in a material with zero resistance. Charge trapping is supposed to be a telltale sign of an insulator. Together with Leiden theoretical physicist Jan Zaanen, Allan’s group found that the phenomenon could unravel a longstanding mystery about charge transport in a family of materials called cuprates. These poorly understood materials have no resistance, even at relatively high temperatures, and are therefore labeled high-temperature superconductors. The mechanism behind those is one of the big mysteries in physics today.
Post link
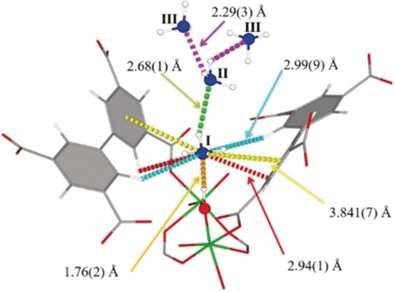
A robust material for the uptake and storage of ammonia at densities that come close to that of the liquefied gas
Handling, storing, and shipping of ammonia requires costly equipment and special precautions because of its inherent corrosiveness and toxicity. Scientists in Manchester, UK, have found that a metal–organic framework, MFM-300(Al), a porous solid, not only effectively filters harmful nitrogen dioxide gas, but it also has outstanding capabilities for ammonia storage. As detailed in the journal Angewandte Chemie, reversible uptake and release of ammonia proceeds by a unique sorption mode.
Ammonia is an essential nitrogen source for plants and it is a basic chemical. This indispensable chemical, which is manufactured on a large scale from atmospheric nitrogen and hydrogen, has been called “bread from air”. But how should this resource be stored and handled? The gaseous or liquefied form is corrosive and toxic. Storing and shipping under pressure or at low temperatures is costly and energy-consuming. Adsorption in porous solids, such as zeolites or metal–organic frameworks—a strategy currently being tested extensively in hydrogen storage—could be an interesting option.
The robust metal–organic framework MFM-300(Al) has been shown to be a potent filter for nitrogen dioxide, which is a harmful pollutant in air. Martin Schröder and his colleagues at the University of Manchester, UK, have now scrutinized MFM-300(Al) for its ability to take up ammonia. They discovered that it could take up gaseous ammonia up to a density that comes close to that of liquid ammonia under ambient conditions. At around zero degrees Celsius it even surpassed this density.
Post link
Extracting energy from a 60 nanometers thin layer
A team of researchers have demonstrated the viability of the direct piezoelectric effect in a thin film Bismuth Ferrite Material for the first time. The work, published in Nanoscale entitles “Direct and Converse Piezoelectric Responses at the Nanoscale from Epitaxial BiFeO3 Thin Films Grown by Polymer Assisted Deposition” which has gained the cover letter of such journal.
[…]
In this particular research, the BFO was scanned in a novel methodology named “Direct Piezoelectric Force Microscopy” DPFM, a new AFM mode invented in 2017
(https://www.nature.com/articles/s41467-017-01361-2 ). The material in this mode is stressed by the AFM tip with nanometric size. The tip applies a force in the range of hundreds of microNewton and measures the generated charge that is created by the material. For the case of BFO material, the piezoelectric characteristics were collected when the tip crosses antiparallel domain configurations, see the following video for a 3D representation of the tip crossing such configuration: https://youtu.be/ir3W2Vk8hCs
Post link
Supercomputer predicts optical and thermal properties of complex hybrid materials
Materials scientists at Duke University computationally predicted the electrical and optical properties of semiconductors made from extended organic molecules sandwiched by inorganic structures.
These types of so-called layered “hybrid organic-inorganic perovskites"—or HOIPs—are popular targets for light-based devices such as solar cells and light-emitting diodes (LEDs). The ability to build accurate models of these materials atom-by-atom will allow researchers to explore new material designs for next-generation devices.
The results appeared online on October 4 in Physical Review Letters.
"Ideally we would like to be able to manipulate the organic and inorganic components of these types of materials independently and create semiconductors with new, predictable properties,” said David Mitzi, the Simon Family Professor of Mechanical Engineering and Materials Science at Duke. “This study shows that we are able to match and explain the experimental properties of these materials through complex supercomputer simulations, which is quite exciting.”
Post link
Graphene controls surface magnetism at room temperature
Typically research has focused on the effects induced by different materials in graphene. Convinced that this is only half the story, Dr Zeila Zanolli turned the tables to look at the proximity effects of graphene on magnetic semiconducting substrates. Using first principles calculations she observes a switching of internal spin alignment from antiferromagnetic to ferromagnetic. Persisting close to room temperature, her findings could find applications in magnetic memories or spin filters.
[…]
In a refreshing change of perspective, theoretical physicist Dr Zeila Zanolli has looked at the proximity effects of graphene on a magnetic semiconducting substrate, finding it to affect the substrate’s magnetism down to several layers below the surface. Her paper was published on 5 October in Physical Review B. She was also one of three recipients of the first MaX Prize for frontier research in computational materials science.
Post link
Polymers: Polyphenylene oxide
Though its technical name is poly(2,6-dimethylphenylene oxide) it is most commonly known as poly(phenylene oxide) or PPO. A condensation polymer, the polymerization of PPO produces water as a byproduct. It is a type of polyphenylene and is often grouped alongside polyphylene ethers as well.
PPO is one of many high-performance polymers known as engineering thermoplastics. These polymers have higher glass transition temperatures, making them temperature resistant. However, these polymers are often more crystalline, and therefore more brittle. In order to help with processing of PPO and to increase the toughness, almost all commercially available forms of the polymer are blended with polystyrene (such as high-impact polystyrene, or HIPS).
Applications of PPO (or, more commonly, blends of PPO and polystyrene) include those shown on the chart above. PPO is often used in applications where a polymer is desired but high heat resistance is also necessary, such as certain structural applications, electronics, and medical equipment (such as sterilizable instruments). Another common usage is in air separation membranes for the generation of nitrogen, an application which uses hollow fibers to create a membrane.
Sources/Further Reading: ( 1 - image 1 ) ( 2 - image 2 ) ( 3 - image 3 ) (4) (5)
Post link
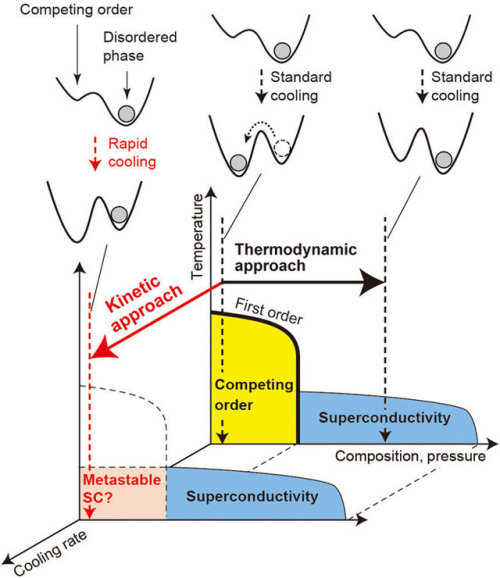
Forcing a metal to be a superconductor via rapid chilling
A team of researchers with the RIKEN Center for Emergent Matter Science and The University of Tokyo, both in Japan, has found a way to force a metal to be a superconductor by cooling it very quickly. In their paper published on the open access site, Science Advances, the group describes their process and how well it worked.
Scientists around the world continue to seek a material that behaves as a superconductor at room temperature—such a material would be extremely valuable because it would have zero electrical resistance. Because of that, it would not increase in heat as electricity passed through it, nor lose energy. Scientists have known that cooling some materials to very cold temperatures causes them to be superconductive. They have also known that some metals fail to do so because they enter a “competing state.” In this new effort, the researchers in Japan have found a way to get one such non-cooperative metal to enter a superconductive state anyway—and to stay that way for over a week.
Post link
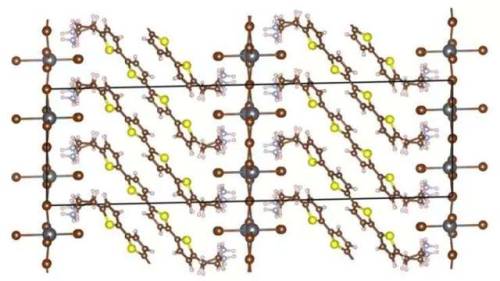
Copper ions flow like liquid through crystalline structures
Materials scientists have sussed out the physical phenomenon underlying the promising electrical properties of a class of materials called superionic crystals. A better understanding of such materials could lead to safer and more efficient rechargeable batteries than the current standard-bearer of lithium ion.
Becoming a popular topic of study only within the past five years, superionic crystals are a cross between a liquid and a solid. While some of their molecular components retain a rigid crystalline structure, others become liquid-like above a certain temperature, and are able to flow through the solid scaffold.
In a new study, scientists from Duke University, Oak Ridge National Laboratory (ORNL) and Argonne National Laboratory (ANL) probed one such superionic crystal containing copper, chromium and selenium (CuCrSe2) with neutrons and X-rays to determine how the material’s copper ions achieve their liquid-like properties. The results appear online on Oct. 8 in the journal Nature Physics.
“When CuCrSe2 is heated above 190 degrees Fahrenheit, its copper ions fly around inside the layers of chromium and selenium about as fast as liquid water molecules move,” said Olivier Delaire, associate professor of mechanical engineering and materials science at Duke and senior author on the study. “And yet, it’s still a solid that you could hold in your hand. We wanted to understand the molecular physics behind this phenomenon.”
Post link
Novel use of NMR sheds light on easy-to-make electropolymerized catalysts
In the world of catalytic reactions, polymers created through electropolymerization are attracting renewed attention. A group of Chinese researchers recently provided the first detailed characterization of the electrochemical properties of polyaniline and polyaspartic acid (PASP) thin films. In AIP Advances, the team used a wide range of tests to characterize the polymers, especially their capacity for catalyzing the oxidation of popularly used materials, hydroquinone and catechol.
This new paper marks one of the first pairings of standard electrochemical tests with nuclear magnetic resonance (NMR) analysis in such an application. “Because these materials can be easily prepared in an electric field and are cost-effective and environmentally friendly, we think they have the potential to be widely used,” said Shuo-Hui Cao, an author on the paper.
Although PASP has shown excellent electrocatalytic responses to biological molecules, newer areas of inquiry have explored the material’s ability to lower the oxidational potential in oxidation-reduction reactions. Reducing the oxidation potential is key for finding further uses for two materials used extensively as raw materials and synthetic intermediates in pharmaceuticals, hydroquinone and catechol.
Post link
Light makes Rice U. catalyst more effective: Halas lab details plasmonic effect that allows catalyst to work at lower energy
Rice University nanoscientists have demonstrated a new catalyst that can convert ammonia into hydrogen fuel at ambient pressure using only light energy, mainly due to a plasmonic effect that makes the catalyst more efficient.
[…]
A study from Rice’s Laboratory for Nanophotonics (LANP) in this week’s issue of Science describes the new catalytic nanoparticles, which are made mostly of copper with trace amounts of ruthenium metal. Tests showed the catalyst benefited from a light-induced electronic process that significantly lowered the “activation barrier,” or minimum energy needed, for the ruthenium to break apart ammonia molecules.
The research comes as governments and industry are investing billions of dollars to develop infrastructure and markets for carbon-free liquid ammonia fuel that will not contribute to greenhouse warming. But the researchers say the plasmonic effect could have implications beyond the “ammonia economy.”
Post link
Chameleon-Like Material Spiked With Boron Helps Bring Brain-Like Computing to Silicon Chips
Chameleon-Like Material Spiked With Boron Comes Closer To Mimicking Brain Cells
In a new study, Texas A&M researchers in the Department of Materials Science and Engineering describe a new material that comes close to mimicking how brain cells perform computations.
Each waking moment, our brain processes a massive amount of data to make sense of the outside world. By imitating the way the human brain solves everyday problems, neuromorphic systems have tremendous potential to revolutionize big data analysis and pattern recognition problems that are a struggle for current digital technologies.
But for artificial systems to be more brain-like, they need to replicate how nerve cells communicate at their terminals, called the synapses.
Post link
Technological ray of hope for the snowboard scene
The first boards for gliding over snow existed as early as 1900, but it was not until 1963 that American surfers brought the feeling of surfing to the snow and developed the original snowboard—the so-called snurfer. A few years later, the snowboard drew the interest of the winter sports industry, and since 1998, snowboarding has been recognized as an Olympic sport.
Chemnitz University of Technology researchers have presented an innovation from the 2020/2021 winter sports season: Together with silbaerg GmbH, a spin-off from the Institute of Lightweight Structures at Chemnitz University of Technology, they have developed a lightweight snowboard that can also be manufactured far more sustainably than comparable boards. This is made possible by a new type of textile fiber, a semi-finished product made of carbon fibers. By using the dry fiber placement process, fiber waste in snowboard production can be reduced by around 60%. “This not only saves costs, but thanks to the board’s sustainable production, its carbon footprint is also significantly reduced,” says Prof. Dr. Holger Cebulla, head of the Chair of Textile Technologies.
Post link
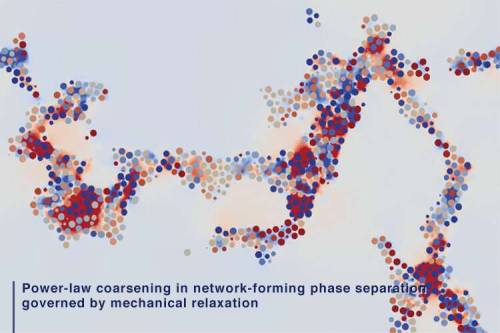
Discovery of a new law of phase separation
Researchers from Institute of Industrial Science at The University of Tokyo investigated the mechanism of phase separation into the two phases with very different particle mobilities using computer simulations. They found that slow dynamics of complex connected networks control the rate of demixing, which can assist in the design of new functional porous materials, like lithium-ion batteries.
According to the old adage, oil and water don’t mix. If you try to do it anyway, you will see the fascinating process of phase separation, in which the two immiscible liquids spontaneously “demix.” In this case, the minority phase always forms droplets. Contrary to this, the researchers found that if one phase has much slower dynamics than the other phase, even the minority phase form complex networks instead of droplets. For example, in phase separation of colloidal suspensions (or protein solutions), the colloid-rich (or protein-rich) phase with slow dynamics forms a space-spanning network structure. The network structure thickens and coarsens with time while having the remarkable property of looking similar over a range of length scales, so the individual parts resemble the whole.
Post link
materialsscienceandengineering:
Scientists break record for highest-temperature superconductor: Experiment produces new material that can conduct electricity perfectly
University of Chicago scientists are part of an international research team that has discovered superconductivity–the ability to conduct electricity perfectly–at the highest temperatures ever recorded.
[…]
Using advanced technology at UChicago-affiliated Argonne National Laboratory, the team studied a class of materials in which they observed superconductivity at temperatures of about minus-23 degrees Celsius (minus-9 degrees Fahrenheit)–a jump of about 50 degrees compared to the previous confirmed record.
Though the superconductivity happened under extremely high pressure, the result still represents a big step toward creating superconductivity at room temperature–the ultimate goal for scientists to be able to use this phenomenon for advanced technologies. The results were published May 23 in the journal Nature; Vitali Prakapenka, a research professor at the University of Chicago, and Eran Greenberg, a postdoctoral scholar at the University of Chicago, are co-authors of the research.
Post link
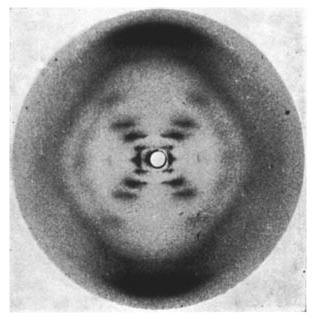
Many of you may recognize this photo of the x-ray diffraction pattern of DNA found by Rosalind Franklin and her PhD student, Raymond Gosling. But, you may wonder how one could figure out from this image that DNA is structured as a double helix and even how x-ray crystallography works.
X-Ray Crystallography
X-ray crystallography is a method of determining the positions and arrangements of atoms in a crystal. Crystals are usually defined to be a highly ordered and repeating microscopic structure of a solid rather than the macroscopic crystals we know like quartz which actually tend to be “polycrystals” because at a microscopic level they do have the highly ordered structure required. Ice is also a polycrystal composed of many smaller ice crystals.
1.) X-ray beams are shot at the crystals
The x-rays interact with electrons of the atoms. This interaction or collision is typically modeled by Thomson scattering where the energy and thus frequency of the x-rays do not change after diffraction. This is similar to light going through a diffraction grating.
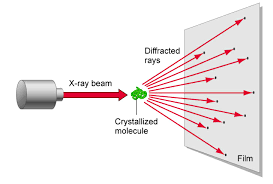
2.) Beam is diffracted
The x-rays are diffracted based on the crystal lattice structure of the substance. This is dependent on the characteristics of the bonds between atoms like the bond angles and bond lengths. Also the spacing between molecules also determines the diffraction.

3.) Diffraction pattern
The diffracted x-rays are light waves so they interfere both constructively and destructively. The resulting intensities of the x-rays are recorded on a screen behind the sample to create a diffraction pattern. The sample is rotated to take more data. After sufficient data is taken a model for the crystal structure for the sample can be developed. With a diffraction pattern an electron density map can be made which depicts the location and size of electron clouds in the substance.

Above is an example of an electron density map.
materialsscienceandengineering:
Giant lasers crystallize water with shockwaves, revealing the atomic structure of superionic ice
Scientists from Lawrence Livermore National Laboratory (LLNL) used giant lasers to flash-freeze water into its exotic superionic phase and record X-ray diffraction patterns to identify its atomic structure for the very first time—all in just a few billionths of a second. The findings are reported today in Nature.
In 1988, scientists first predicted that waterwould transition to an exotic state of matter characterized by the coexistence of a solid lattice of oxygen and liquid-like hydrogen—superionic ice—when subjected to the extreme pressures and temperatures that exist in the interior of water-rich giant planets like Uranus and Neptune. These predictions remained in place until 2018, when a team led by scientists from LLNL presented the first experimental evidence for this strange state of water.
Now, the LLNL scientists describe new results. Using laser-driven shockwaves and in-situ X-ray diffraction, they observe the nucleation of a crystalline lattice of oxygen in a few billionths of a second, revealing for the first time the microscopic structure of superionic ice.
Post link
materialsscienceandengineering:
Oregon scientists drill into white graphene to create artificial atoms: Patterned on a microchip and working in ambient conditions, the atoms could lead to rapid advancements in new quantum-based technology
By drilling holes into a thin two-dimensional sheet of hexagonal boron nitride with a gallium-focused ion beam, University of Oregon scientists have created artificial atoms that generate single photons.
[…]
The artificial atoms - which work in air and at room temperature - may be a big step in efforts to develop all-optical quantum computing, said UO physicist Benjamín J. Alemán, principal investigator of a study published in the journal Nano Letters.
“Our work provides a source of single photons that could act as carriers of quantum information or as qubits. We’ve patterned these sources, creating as many as we want, where we want,” said Alemán, a member of the UO’s Material Science Institute and Center for Optical, Molecular, and Quantum Science. “We’d like to pattern these single photon emitters into circuits or networks on a microchip so they can talk to each other, or to other existing qubits, like solid-state spins or superconducting circuit qubits.”
Post link
Today’s FYFD video tells a story I’ve wanted to share for a couple of years now. It’s about the life and work of Agnes Pockels, a woman born in the mid-nineteenth century who, despite a lack of formal scientific training, made major contributions to the understanding of surface tension and to the experimental apparatuses and methodologies used in surface chemistry in general. She accomplished all of this not in a scientific lab, but from her kitchen.
Pockels’ story is one of curiosity, determination, and meticulous scientific inquiry. Chances are that you’ve never heard of her, but you really should. Check out the full video below to learn more! (Image and video credit: N. Sharp)
Post link