#proteins

(Image caption: Protein expression in the mouse brain for mass spectrometry analysis, visualized by fluorescence microscopy. Blue shows the outline of the brain. Green and magenta are selectively tagged proteins)
New technique identifies proteins in the living brain
For the first time, researchers have developed a successful approach for identifying proteins inside different types of neurons in the brain of a living animal.
Led by Northwestern University and the University of Pittsburgh, the new study offers a giant step toward understanding the brain’s millions of distinct proteins. As the building blocks of all cells including neurons, proteins hold the keys to better understanding complex brain diseases such as Parkinson’s and Alzheimer’s, which can lead to the development of new treatments.
The study was published in the journal Nature Communications.
In the new study, researchers designed a virus to send an enzyme to a precise location in the brain of a living mouse. Derived from soybeans, the enzyme genetically tags its neighboring proteins in a predetermined location. After validating the technique by imaging the brain with fluorescence and electron microscopy, the researchers found their technique took a snapshot of the entire set of proteins (or proteome) inside living neurons, which can then be analyzed postmortem with mass spectroscopy.
“Similar work has been done before in cellular cultures. But cells in a dish do not work the same way they do in a brain, and they don’t have the same proteins in the same places doing the same things,” said Northwestern’s Yevgenia Kozorovitskiy, senior author of the study. “It’s a lot more challenging to do this work in the complex tissue of a mouse brain. Now we can take that proteomics prowess and put it into more realistic neural circuits with excellent genetic traction.”
By chemically tagging proteins and their neighbors, researchers can now see how proteins work within a specific, controlled area and how they work with one another in a proteome. Along with the virus carrying the soybean enzyme, the researchers also used their virus to carry a separate green fluorescent protein.
“The virus essentially acts as a message that we deliver,” Kozorovitskiy said. “In this case, the message carried this special soybean enzyme. Then, in a separate message, we sent the green fluorescent protein to show us which neurons were tagged. If the neurons are green, then we know the soybean enzyme was expressed in those neurons.”
Kozorovitskiy is the Soretta and Henry Shapiro Research Professor of Molecular Biology, an associate professor of neurobiology in Northwestern’s Weinberg College of Arts and Sciencesand a member of the Chemistry of Life Processes Institute. She co-led the work with Matthew MacDonald, an assistant professor of psychiatry at the University of Pittsburgh Medical Center.
Protein targeting plays catch-up
While genetic targeting has completely transformed biology and neuroscience, protein targeting has woefully lagged behind. Researchers can amplify and sequence genes and RNA to identify their exact building blocks. Proteins, however, cannot be amplified and sequenced in the same manner. Instead, researchers have to divide proteins into peptides and then put them back together, which is a slow and imperfect process.
“We have been able to gain a lot of traction with genetic and RNA sequencing, but proteins have been out of the loop,” Kozorovitskiy said. “Yet everyone recognizes the importance of proteins. Proteins are the ultimate effectors in our cells. Understanding where proteins are, how they work and how they work relative to each other is really important.”
“Mass spectroscopy-based proteomics is a powerful technique,” said Vasin Dumrongprechachan, a Ph.D. candidate in Kozorovitskiy’s laboratory and the paper’s first author. “With our approach, we can start mapping the proteome of various brain circuits with high precision and specificity. We can even quantify them to see how many proteins are present in different parts of neurons and the brain.”
Next step: Better understanding brain diseases
Now that this new system has been validated and is ready to go, the researchers can apply it to mouse models for disease to better understand neurological illnesses.
“We are hoping to extend this approach to start identifying the biochemical modifications on neuronal proteins that occur during specific patterns of brain activity or with changes induced by neuroactive drugs to facilitate clinical advances,” Dumrongprechachan said.
“We look forward to taking this to models related to brain diseases and connect those studies to postmortem proteomics work in the human brain,” Kozorovitskiy said. “It’s ready to be applied to those models, and we can’t wait to get started.”
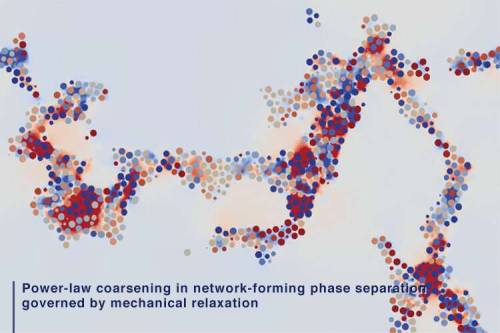
Discovery of a new law of phase separation
Researchers from Institute of Industrial Science at The University of Tokyo investigated the mechanism of phase separation into the two phases with very different particle mobilities using computer simulations. They found that slow dynamics of complex connected networks control the rate of demixing, which can assist in the design of new functional porous materials, like lithium-ion batteries.
According to the old adage, oil and water don’t mix. If you try to do it anyway, you will see the fascinating process of phase separation, in which the two immiscible liquids spontaneously “demix.” In this case, the minority phase always forms droplets. Contrary to this, the researchers found that if one phase has much slower dynamics than the other phase, even the minority phase form complex networks instead of droplets. For example, in phase separation of colloidal suspensions (or protein solutions), the colloid-rich (or protein-rich) phase with slow dynamics forms a space-spanning network structure. The network structure thickens and coarsens with time while having the remarkable property of looking similar over a range of length scales, so the individual parts resemble the whole.
Post link
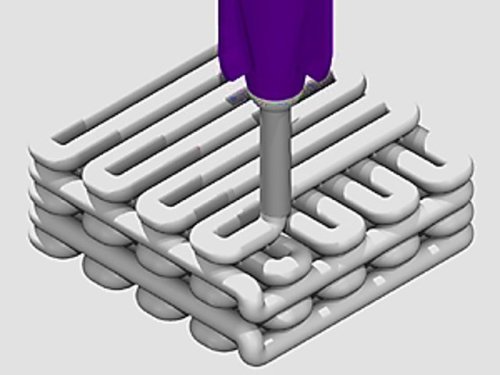
Advanced biomaterials with silk fibroin-bioactive glass to engineer patient-specific 3-D bone grafts
The complex architecture of bone is challenging to recreate in the lab. Therefore, advances in bone tissue engineering (BTE) aim to build patient-specific grafts that assist bone repair and trigger specific cell-signaling pathways. Materials scientists in regenerative medicine and BTE progressively develop new materials for active biological repair at a site of defect post-implantation to accelerate healing through bone biomimicry.
Rapidly initiation of new bone formation at the site of implantation is a highly desirable feature in BTE, and scientists are focused on fabricating grafts that strengthen the material-bone interface after implantation. Bioactive glass can bond with bone minutes after grafting, and silk fibroin, a natural fibrous protein has potential to induce bone regeneration. Hybrid materials that exploit these properties can combine the osteogenic potential and the load-bearing capacity for potential applications in large-load bone defect models.
In a recent study, Swati Midha and co-workers developed a novel 3-D hybrid construct using silk-based inks with different bioactive glass compositions integrated to recreate a bone-mimetic microenvironment that supports osteogenic differentiation of bone marrow mesenchymal stem cell (BMSC) lines in the lab. Now published in Biomedical Materials, IOP Science, the scientists used direct writing instruments to produce the silk fibroin-gelatin-bioactive glass scaffolds (SF-G-BG). The results delivered appropriate cues to regulate the development of customized 3-D human bone constructs in vitro.
Post link
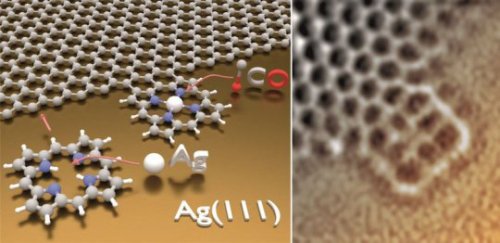
How porphyrin may enhance graphene
Porphyrins, the same molecules that convey oxygen in hemoglobin and absorb light during photosynthesis, can be joined to the material of the future, graphene, to give it new properties. This was recently shown by a team of scientists at the Technical University of Munich, in which a Spanish researcher also participated. The resulting hybrid structures could be used in the field of molecular electronics and in developing new sensors.
At the moment, it is difficult to find a material that attracts as much attention from scientists and engineers as graphene, which is made up of a layer of carbon atoms arrange in a hexagonal structure. It is flexible, extremely thin and clear, while being highly resistant and a conductor of electricity – ideal requirements for a number of uses, especially in the field of electronics.
However, using graphene to capture solar energy or as a gas sensor requires specific properties which it lacks, although it can acquire them by addition or functionalisation with certain molecules.
A team of researchers from the Technical University of Munich (TUM), led by Professor Wilhelm Auwärter, has succeeded in bonding an important biochemical group to the graphene sheet: porphyrins, protein rings which are part of chlorophyll, essential for photosynthesis in plants, and hemoglobin, which is responsible for conveying oxygen in animals’ blood.
Post link
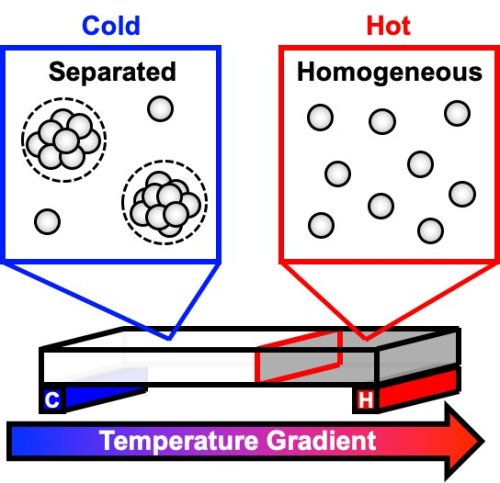
New model describes phase separation that spoils antibody solutions
A new mathematical model describes how highly concentrated antibody solutions separate into different phases, similar to an oil and water mixture. This separation can reduce the stability and shelf-life of some drugs that use monoclonal antibodies, including some used to treat autoimmune diseases and cancer. A team of scientists from Penn State and MedImmune, LLC (now AstraZeneca) investigated the thermodynamics and kinetics, the relationships between temperature, energy, and the rates of chemical reactions, of the phenomenon using an innovative method that allows for the rapid study of multiple samples at once. A paper describing their model appears July 22, 2019, in the journal Proceedings of the National Academy of Sciences.
Many drugs today are stored as solids and dissolved in IV bags for delivery to patients, but the pharmaceutical industry has been moving toward drugs that can be stored as liquids and given via a shot. Some of these drug solutions, like those used to treat autoimmune diseases and some cancers, contain high concentrations of monoclonal antibodies—proteins that attach to foreign substances in the body, like bacteria and viruses, flagging them for destruction by the patient’s immune system.
Post link
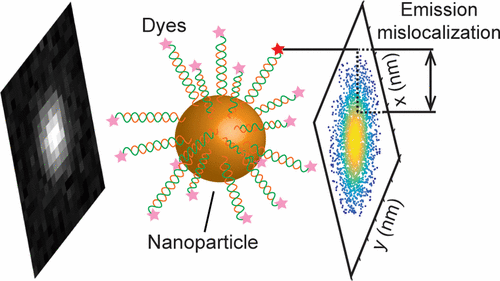
Nanoparticles can help scientists brighten their research—but they also can throw off microscopic measurements
Gold nanoparticles brighten the fluorescent dyes researchers use to highlight and study proteins, bacteria and other cells, but the nanoparticles also introduce an artifact that makes the dye appear removed from the target it’s illuminating.
Now, a University of Michigan team has determined how to account for the discrepancy between where the fluorescent dye appears to be and where its actual position is.
When researchers want to understand how proteins interact with each other, how bacteria function or how cells grow and divide, they often use fluorescent dyes. This microscopy approach can be further enhanced with nanoparticles. But an artifact introduced by the nanoparticles makes the dye appear in the microscope as far as 100 nanometers removed from the proteinor bacteria to which it is directly bound.
This “scooching effect” presents a problem: 100 nanometers may seem like an infinitesimal measurement, but if a protein is itself only a nanometer in length, a researcher might not be able to tell whether a protein is interacting with another protein or just gazing at it from the equivalent of the opposite end of a football field.
Post link
Mussels inspire surgical glue invention
UC Berkeley engineer Phillip Messersmith is happy to be learning lessons from a lowly mollusk, with the expectation that the knowledge gained will enable him and fellow physicians to prevent deaths among their youngest patients — those who haven’t been born yet.
For full story, visit: http://news.berkeley.edu/2016/06/30/f…
Equipped with new funding from the National Institutes of Health and a partnership with a physician known as the “father of fetal surgery,” Messersmith is making better glues for medical procedures inside the body, applying what he and others before him have learned about underwater superglue-making techniques that have been developed and elaborated upon through eons of evolution by mussels, a brainless bivalve.
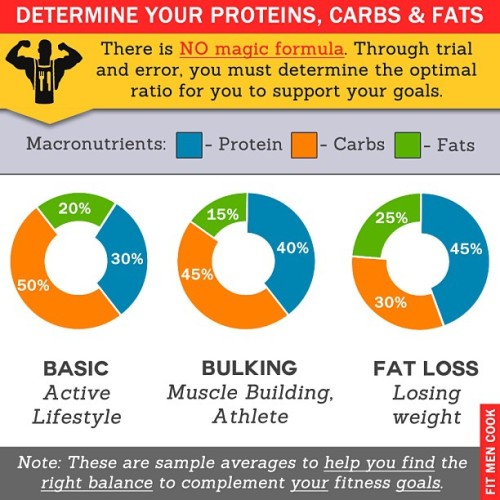
Proteins in Materials Applications
Proteins play a vital role in countless biological proccess, including allowing our cells to reproduce (DNA replication), but they can also be used outside living bodies to generate biomaterials that can be used in diverse applications. Such biomaterials would be biocompatible by nature, drawing interest in the biomedical field, and are more sustainable than the common polymers that exist today as they are also typically biodegradable. In addition, proteins are made of discrete amino acid sequences, thus allowing for easy modification based on the numerous ways these can be combined.
Silk, wool, and leather are all technically protein-based materials used to create textiles for millenia. Well known proteins include silk (silk, by itself, is not a protein, but the core of silk is fibroin), collagen (the most abundant structural protein in mammalian tissues), and keratin (in hair and wool, but also the claws of reptiles and the feathers of birds) and these proteins typically have excellent strength and elasticity. In modern-day materials applications, proteins are typically processed one of three ways: into a gel, a film, or fibers.
For a few select examples: Spider silk is an example of a well-known protein that is naturally spun into fibers known as nanofibrils. Moreover, amyloid fibrils (formed from misfolded proteins) have been used in biosensing applications, for their conductivity and mechanical strength, and for water purification. They have been integrated into aerogels, which are of interest in applications ranging from thermal and acoustic insulation to water purification, to catalysis, and membranes, which have also been used for water purification by helping to remove heavy metal ions.
Protein-based materials are often created as composites, combined with other materials to produce the desired functionality. The aerogel reference above was functionalized with gold nanoparticles. The membranes for water purification were made with activated carbon as well.
Sources/Further reading: ( 1 - image 2 source ) (2) (3) (4) ( 5 images 1 and 3 )
Post link
It Looks, Smells, And Feels Like Real Leather–But It’s Grown In A Lab
Modern Meadow is trying to revolutionize the multi-billion dollar leather industry.
Researchers create self-assembled logic circuits from proteins
In a proof-of-concept study, researchers have created self-assembled, protein-based circuits that can perform simple logic functions. The work demonstrates that it is possible to create stable digital circuits that take advantage of an electron’s properties at quantum scales.
One of the stumbling blocks in creating molecular circuits is that as the circuit size decreases the circuits become unreliable. This is because the electrons needed to create current behave like waves, not particles, at the quantum scale. For example, on a circuit with two wires that are one nanometer apart, the electron can “tunnel” between the two wires and effectively be in both places simultaneously, making it difficult to control the direction of the current. Molecular circuits can mitigate these problems, but single-molecule junctions are short-lived or low-yielding due to challenges associated with fabricating electrodes at that scale.
“Our goal was to try and create a molecular circuit that uses tunneling to our advantage, rather than fighting against it,” says Ryan Chiechi, associate professor of chemistry at North Carolina State University and co-corresponding author of a paper describing the work.
Post link
RG @okiroi_s.a: Το σαπούνι OKIRÒI με γάλα γαϊδούρας, ιδανικό για πρόσωπο και σώμα, λόγω της σύνθεσής του που είναι πλούσια σε πρωτείνες, φωσφολιπίδια, ceramides και υδατάνθρακες, συμβάλει δραστικά στην ίαση δερματικών παθήσεων όπως είναι η ψωρίαση και το έκζεμα και στην επούλωση μικροπληγών (ακμή, ανεμοβλογιά), ρυθμίζοντας τη φυσιολογική μεταβολική δραστηριότητα των κυττάρων.
#OKIRÒI #donkey #milk #soap #bar, ideal for #face and #body, because of its rich #composition in #proteins, #phospholipids, #ceramides and #carbohydrates, helps #cure #skin #diseases such as #psoriasis and #eczema and #heals minor wounds (#acne, #chicken pox), regulating the normal #metabolic #activity of #cells. #regramapp
Post link
Hello you, I thought about a new category here. Ready for factly friday :)?
Means, each Friday I’ll share some cool facts for you.
If you have any specific questions, just comment below.
Oh and I case you know people complaining about that you can’t get enough proteins on a vegan diet, just share this ❣️.
Thank you for spreading the love.
#factlyfriday #fridayfacts #proteins #proteinsource #veganprotein #plantproteins #fitvegan #healthyeating #gesundleben #mixdeinglück #legumes #lentils #tempeh #soy (at Planet Earth)
https://www.instagram.com/p/B7JXhinlaSn/?igshid=1vos8acvk7rlr
Post link
Plantibodies and Plant-Derived Edible Vaccines
Throughout history, humans have used plants in the treatment of disease. This includes more traditional methods involving direct consumption with minimal preparation involved and the extraction of compounds for use in modern pharmaceuticals. One of the more recent methods of using plants in medicine involves the synthesis and application of plantibodies and plant produced antigens. These are recombinant antibodies and antigens respectively, which have been produced by a genetically modified plant (1, 2).
Antibodies are a diverse set of proteins which serve the purpose of aiding the body in eliminating foreign pathogens. They are secreted by effector B lymphocytes which are a type of white blood cell that circulate throughout the body. An antigen is a molecule or a component of a molecule, such as a protein or carbohydrate, which can stimulate an immune response. The human body is capable of producing around 1012 different types of antibodies, each of which can bind to a specific antigen or a small group of related motifs (3). When an antibody encounters the antigen of a foreign pathogen to which it has high affinity, it binds to it which can disable it or alert the immune system for its destruction (4).
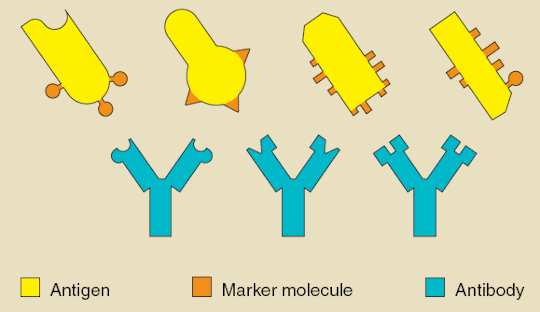
Plants do not normally produce antibodies and thus must be genetically modified to produce plantibodies as well as foreign protein antigens. Plantibodies produced in this manner function the same way as the antibodies native to the human body (1). The main ways to do this are to stably integrate foreign DNA into a host cell and place it into a plant embryo resulting in a permanent change of the nuclear genome, or to induce transient gene expression of the specified protein (5). In both cases, the genetic material introduced to the plant codes for the protein of choice. Several of the methods used to induce permanent transgene expression include agrobacterium-mediated transformation, particle bombardment using a gene gun, or the transformation of organelles such as chloroplasts. Transient transgene expression can be done using plant viruses as viral vectorsoragroinfiltration (2). Once the genetic material has been inserted, the specified protein is produced via the plant endomembrane and secretory systems, after which it can be recovered through purification of the plant tissue to be used for injection (1). The production of these proteins can also be directed to specific organs of the plant such as the seeds using targeting signals (2). Stable integration techniques are generally used for more large scale production and when the gene in question has a high level of expression, while transient techniques are used to produce a greater yield in the short term (5).

Figure 2: A gene gun being used to introduce genetic material into the leaves of a plant.
Now how can plantibodies and plant produced antigens help us as humans? The primary purpose of producing plantibodies is for the treatment of disease via immunotherapy. Immunotherapy is a method of treatment in which one’s immune response to a particular disease is enhanced. Specific plantibodies can be produced in order to target a particular disease and then be applied to patients via injection as a means of treatment (6). Doing so provides a boost to the number of antibodies against the targeted disease in the patient’s body which helps to enhance their immune system response against it. An example of this is CaroRx, the first clinically tested plantibody which has the ability to bind to Streptococcus mutans. CaroRx has been shown to be effective in the treatment of tooth decay caused by this species of bacteria (1). More recently, a plantibody known as ZMapp has shown potential in the treatment of Ebola. A study by Qiu et al showed that when administered up to 5 days after the onset of the disease, 100% of rhesus macaques that were administered the drug were shown to have recovered from its effects while all of the control group animals perished as a result of the disease (7). In addition, it has been experimentally administered to some humans who later recovered from the disease, although its role in their recovery was not fully ascertained (8).
Plant produced antigens on the other hand can be used to produce oral vaccines (9). Vaccines are typically biological mixtures containing a weakened pathogen and its antigens. Injection of this results in priming of the body’s adaptive immune system against the particular pathogen so that it can more easily recognize and respond to the threat in the future (4). By producing the antigens of targeted pathogens in plants through transgenic expression, edible vaccines can be created if the plant used is safe to eat. Tobacco, potato, and tomato plants have typically been used in past attempts to create them, showing success in both animal studies and a number of human trials. The advantages of using an oral vaccine include ease of administration and lower costs since specialised personel are not required for administration (9). In addition, oral vaccines are more effective in providing immunity against pathogens at mucosal surfaces as they can be directly applied to the gastrointestinal tract (1). The primary issue with the usage of oral vaccines is that protein antigens must avoid degradation in the stomach and intestines before they can reach the targeted sites in the body. Several solutions to this dilemma include using other biological structures such as liposomesandproteasomes as a means of delivery. This helps to prevent the proteins from being degraded by digestive enzymes and the acidic environment of the stomach before they can reach their destination (1, 9).

There are a number of advantages to using these plant based pharmaceuticals. First of all, they can be produced on a large scale at a relatively low cost through agriculture and are convenient for long-term storage due to the resiliency and size of plant seeds (5). There is also a low risk of contamination by mammalian viruses, blood borne pathogens, and oncogenes which can remove the need for expensive removal steps (1). In addition, purification steps can be skipped if the plants used are edible and ethical problems that come with animal production can be avoided (5). The disadvantages include the potential for allergic reactions to plant antigens and contamination by pesticides and herbicides. There is also the possibility of outcrossing of transgenic pollen to weeds or related crops which would lead to non-target crops also expressing the pharmaceutical.This could lead to public concern along with the potential that other species which ingest these plants may be negatively affected (9). While plantibodies and plant produced antigens have not yet been extensively tested in clinical trials, going forward they represent a new treatment option with great promise.
References
1. Jain P, Pandey P, Jain D, Dwivedi P. Plantibody: An overview. Asian journal of Pharmacy and Life Science. 2011 Jan;1(1):87-94.
2. Stoger E, Sack M, Fischer R, Christou P. Plantibodies: applications, advantages and bottlenecks. Current Opinion in Biotechnology. 2002 Apr 1;13(2):161-166.
3. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th Edition. New York: Garland Science; 2002.
4. Parham P. The immune system. 4th Edition. New York: Garland Science; 2014.
5. Ferrante E, Simpson D. A review of the progression of transgenic plants used to produce plantibodies for human usage. J. Young Invest. 2001;4:1-0.
6. Smith MD. Antibody production in plants. Biotechnology advances. 1996 Dec 31;14(3):267-81.
7. Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014 Aug 29.
8. Sneed A. Know the Jargon. Scientific american. 2014 Dec 1;311(6):24-24.
9. Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends in plant science. 2001 May 1;6(5):219-26.
Post link
Please stop using healthy as a synonym for low calorie. They are not the same thing. Thanks.